4805
Initial experience using combined quantitative susceptibility mapping and quantitative bold oxygen level dependent imaging (QSM+qBOLD) oxygen extraction fraction for evaluation of acute ischemic stroke1Radiolgy, Tongji Hospital, Tongji Medical College, HUST, Wuhan, China, 2Radiolgy, Weill Cornell Medical College, NewYork, NY, United States, 3Biomedical Engineering, Cornell University, Ithaca, NY, United States
Synopsis
Oxygen extraction fraction (OEF) reflects tissue oxygen consumption, which is very useful to predict the outcome of ischemic stroke in metabolic level. In this work, we evaluate a combined quantitative susceptibility mapping (QSM) and quantitative bold oxygen level dependent (qBOLD) method for measuring OEF based on MRI multi echo gradient echo (GRE) imaging in 11 acute ischemic stroke patients. OEF maps displayed various patterns both in the lesion and in the ASL-CBF/DWI mismatch area, consistent with previous PET studies. OEF with a heterogeneous increase within the lesion or in the CBF/DWI mismatch area may represent salvageable ischemic tissue, while OEF decrease may suggest irreversible infarct.
Background and purpose: Oxygen extraction fraction (OEF) can reflect the condition of oxygen consuming in tissue, which is very useful to predict the outcome of ischemic stroke in metabolic level. Although positron emission tomography (PET) is considered the gold standard for OEF measurement, it is invasive, time consuming, and expensive, limiting its clinical use. In this work, we show our initial experience using a combined quantitative susceptibility mapping (QSM) and quantitative bold oxygen level dependent (qBOLD) analysis1 of multi echo gradient echo (GRE) data for measuring OEF in a cohort of acute ischemic stroke patients.
Methods: 11 patients with acute ischemic stroke underwent 3D arterial spin labeling (ASL), 3D multi-echo gradient echo (GRE) as well as conventional DWI scanning on a clinical 3T scanner (GE MR Discovery 750 3.0T). The time interval between stroke onset and MRI examination ranged between 6 and 48 hours, and all lesions were located in unilateral middle cerebral artery territory. Cerebral blood flow (CBF) were generated from ASL, and QSM reconstructed from GRE using morphology enabled dipole Inversion (MEDI)2. Then OEF maps were generated using a combined QSM and qBOLD based CMRO2 mapping method1. ROI analysis was performed on registered DWI and CBF images to delineate the stroke lesion, contralateral mirror area, as well as CBF/DWI mismatch area when present.
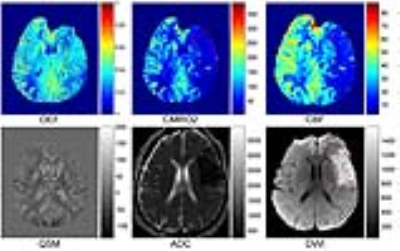
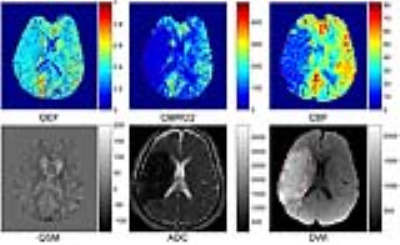
Results: All 11 acute ischemic stroke patients showed acceptable OEF image quality with 6 showing a mismatch between CBF and DWI. Compared to the contralateral mirror side, the lesion area in OEF maps showed diverse manifestation, both surviving tissue within the lesion with similar or higher OEF values (Figure 1, 2) and tissue with lower OEF values. The mismatch area showed lower or equivalent OEF value as compared to the mirror side (Figure 3). The lesion area and mismatch area all have a decrease on CMRO2 maps (Figure 1, 2).
Discussion: Based on this combined QSM and qBOLD model, we found various OEF patterns in lesion area (DWI hyperintense) in acute ischemic stroke patients, the surviving tissue within the lesion can have high OEF (Figure 1), while the DWI positive area with OEF decrease usually suggests irreversible infarct, which is consistent with previous PET studies3 . The mismatch between CBF and DWI defined in this study can represent benign oligemia or ischemic penumbra (Figure 2), while lower OEF in mismatch area may suggest irreversible damage. Further research is needed including a comparison with PET. The CMRO2 decrease in our cases may have an underestimation because of the underestimation of CBF in ischemic stroke.
Conclusion: The preliminary results in this work show the feasibility of evaluating OEF using a combined QSM and qBOLD model. It may be useful in defining the status of ischemic tissue in acute ischemic stroke patients, can help clinical therapy strategy making.
Acknowledgements
This work was supported by NIH grant R21EB024366, R21EY027568, R01NS095562, and S10OD021782.References
1. Cho, J., et al., Optimal quantitative mapping of Cerabral Metabolic Rate of Oxygen (CMRO2) by combining quantitative susceptibility mapping (QSM)-based method and quantitative BOLD (qBOLD). In Proceedings of the 25th Annual Meeting of ISMRM. Hawaii, USA, Abstract 1110.
2. Wang, Y. and T. Liu, Quantitative susceptibility mapping (QSM): Decoding MRI data for a tissue magnetic biomarker. Magn Reson Med, 2015. 73(1): p. 82-101.
3. Guadagno, J.V., et al., The diffusion-weighted lesion in acute stroke: heterogeneous patterns of flow/metabolism uncoupling as assessed by quantitative positron emission tomography. Cerebrovasc Dis, 2005. 19(4): p. 239-46.
Figures