4803
Influences of smoking on cerebral arteriolar vasomotor function: evaluation by using magnetic resonance signal fluctuation1Graduate School of Health Sciences, Hokkaido University, Sapporo, Japan, 2Faculty of Health Sciences, Hokkaido University, Sapporo, Japan
Synopsis
The vasodilation and vasoconstriction properties of cerebral arterioles (arteriolar vasomotor function) would be a biomarker of early diagnosis of dementia. Although the vasodilation ability has been studied by using vasodilators such as Diamox, these vasodilators cause non-natural extreme vasodilation. Focusing on the natural arteriolar vasomotion induced by respiratory variation of blood CO2, we have reported a method to evaluate cerebral arteriolar vasomotor function by spectral analysis of fluctuation of venous MRI signal. In this study, we improved our method and applied it to young smokers, and demonstrated the degeneration of arteriolar vasomotor function after a few years of chronic smoking.
Purpose
The vasodilation and vasoconstriction properties of cerebral arterioles (cerebral arteriolar vasomotor function) would be a biomarker of early diagnosis of dementia.1,2 Although the vasodilation ability has been studied by using vasodilators such as Diamox, these vasodilators cause non-natural extreme vasodilation and are challenging to patients. Focusing on the natural arteriolar vasomotion induced by respiratory variation of blood CO2, we have reported a drag-administration-free method to evaluate cerebral arteriolar vasomotor function in situ by spectral analysis of temporal fluctuation of venous MRI signal.3 Although the spectral purification of the respiratory fluctuation of MRI signal is crucial in our method, aliasing and line broadening accompanied by spectral analysis cause the contamination of other physiological components such as cardiac pulsation. In this study, we addressed these problems and improved our method by observing aliased cardiac frequencies and using a hamming window to sharpen each spectral component. In order to evaluate the influence of smoking, which is a risk factor of dementia, on arteriolar vasomotor function, we applied this improved method to young non-smokers and smokers.Materials and Methods
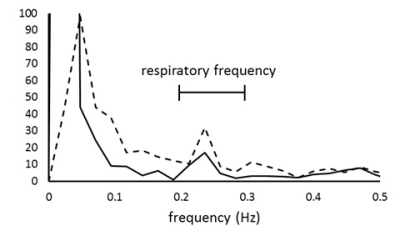
A single slice perpendicular to the superior sagittal sinus was imaged for 45 s by using spin-echo echo-planar imaging (SE-EPI, TR = 250 ms, TE = 30 ms) at 3 T for 7 non-smokers (male, aged 22.6 ± 1.1 years) and 6 smokers (male, aged 24.7 ± 2.2 years, smoking history 4.3 ± 2.1 years). This time series of imaging was repeated for 5 slice thicknesses (7-15 mm). Respiratory rate during scan was fixed at 15 times per minute by a metronome. To investigate the chronic and acute influences of smoking, smokers were imaged after abstaining from smoking for more than 8 hours (pre-smoking) and immediately after smoking (post-smoking) respectively. The time courses of MR signal of superior sagittal sinus were Fourier-transformed for each slice thickness. Because the spectrum at respiratory frequencies was contaminated by line broadening of low frequency component which represents the default mode network, a hamming window was used to reduce this contamination (Fig.1). The spectral fluctuation intensity (SFI) at respiratory frequencies (0.2–0.3 Hz) was obtained for each slice thickness. As aliasing of harmonics of the cardiac pulsation frequency component contaminates the respiratory frequency component, the SFIs which suffers from this contamination due to aliasing were excluded from further analysis by observing the cardiac frequency which can be aliased in respiratory frequencies. SFI versus average signal intensity of superior sagittal sinus was plotted and regression line was drawn for each volunteer. The MR signal of venous blood is written by
$$$S=C\cdot{e^{-R_{2}\cdot{TE}}}\space\space\space\space\space\space\space\space\space\space\space\space\space\space\space\space\space\space\space\space\space\space\space\space\space\space\space\space\space\space$$$(1),
where C is the proportional constant, R2 is a relaxation rate as given by
$$$R_{2}=C_{1}\cdot\left(1-Y\right)^{2}+C_{2}\space\space\space\space\space\space\space\space\space\space\space\space\space\space$$$(2),
where C1、C2 are the constants and Y is the venous blood oxygenation. Using Eqs.1 and 2, the following equation is obtained.
$$$\frac{ΔS}{S}=2C_{1}\cdot\left(1-Y\right)\cdot{TE}\cdot{ΔY_{r}}\space\space\space\space$$$(3),
The right side of Eq. 3 (ΔS/S) is the slope of the regression line of the plot of SFI versus average signal intensity. The respiratory fluctuation of blood oxygenation (ΔYr), which reflects the cerebral arteriolar vasomotor function, can be calculated by substituting the values of C1 (59)4 and Y (0.66).5 Then the values of ΔYr of non-smokers and smokers were compared.
Results and Discussion
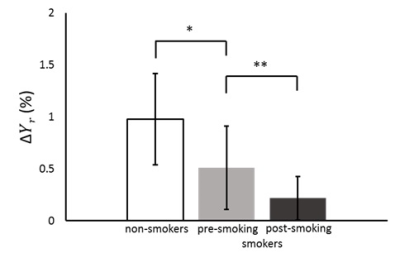
The ΔYr of smokers at pre-smoking (0.7 ± 0.3%) significantly decreased from that of non-smokers (1.2 ± 0.4%) as shown in Fig.2 (p<0.05). This result shows the degeneration of cerebral arteriolar vasomotor function due to chronic smoking and supports the pathological consequence that the chronic smoking is a high risk factor of arteriosclerosis.6 The influence of chronic smoking has been studied for volunteers of more than 30 years old, and we first clarified the influence of chronic smoking of young people of 20s by our drag-administration-free method. Another significant decrease in ΔYr was also found from pre-smoking (0.7 ± 0.3%) to post-smoking (0.3 ± 0.2%) as shown in Fig.2 (p<0.01), which agreed with a Doppler ultrasound study by using carbogen.7 This phenomenon is explained by an acute effect that nicotine makes arterioles vasodilate so as to diminish the vasodilation effect of CO2,7 and hence the ΔYr decreases. We successfully demonstrated the degeneration of cerebral arteriolar vasomotor function due to chronic and acute smoking by our method to elucidate in-situ vasomotion.Conclusion
We improved our drag-administration-free method and demonstrated how the cerebral arteriolar vasomotor function of young volunteers is influenced by chronic and acute smoking. Deterioration of arteriolar vasomotor function may appear in natural vasomotion more clearly than in non-natural extreme vasodilation caused by the administration of vasodilators.Acknowledgements
No acknowledgement found.References
1. Weller RO, Boche D, Nioll JA. Microvascular changes and cerebral amyloid angiopathy in Alzheimer’s disease and their potential impact on therapy. Acta Neuropathol. 2009;11(1):87-102.
2. Kalaria RN. Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutr Rev. 2010;68(2):74-87.
3. Tang M, Nishi K, Yamamoto T. Analysis of Fluctuation in Cerebral Venous Oxygenation Using MR Imaging: Quantitative Evaluation of Vasomotor Function of Arterioles. Magn Reson Med Sci. 2017;16(1):45-53.
4. Qin Q, Grqac K van Zijl PC. Determination of whole-brain oxygen extraction fractions by fast measurement of blood T2 in the jugular vein. Magn Reson Med. 2011;65(2):471-479.
5. Barhoum S, Rodqrs XB Lanqham M, et al. Comparison of MRI methods for measuring whole-brain venous oxygen saturation. Magn Reson Med. 2015;73(6):2122-2128.
6. Boleqo C, Poli A, Paoletti R. Smoking and gender. Cardiovasc Res. 2002;53(3):586-576.
7. Terborg C, Brmer S, Weiller C, et al. Short-term effect of cigarette smoking on CO2-induced vasomotor reactivity in man: A study with near-infrared spectroscopy and tanscranial Doppler sonography. J Neurol Sci. 2002;205(1):15-20.
Figures