4800
Prominent vessels on quantitative susceptibility maps indicate microvascular pathology after experimental cerebral ischemia and reperfusionMarkus Vaas1, Andreas Deistung2,3,4, Jürgen R Reichenbach2,5, Annika Keller6, Anja Kipar7, and Jan Klohs1
1Institute for Biomedical Engineering, University of Zurich and ETH Zurich, Zurich, Switzerland, 2Institute of Diagnostic and Interventional Radiology, University Hospital Jena, Jena, Germany, 3Department of Neurology, Essen University Hospital, Essen, Germany, 4Erwin L. Hahn Institute for Magnetic Resonance Imaging, University Duisburg-Essen, Essen, Germany, 5Michael Stifel Center for Data-driven and Simulation Science Jena, Friedrich Schiller University Jena, Jena, Germany, 6Division of Neurosurgery, University Hospital Zurich, Zurich, Switzerland, 7Institute of Veterinary Pathology, University of Zurich, Zurich, Switzerland
Synopsis
We tested the utility of quantitative susceptibility mapping (QSM) to assess vascular abnormalities in a mouse model of experimental stroke. We acquired high resolution gradient echo data of mice at different time points after ischemia/reperfusion for computation of susceptibility maps. Prominent vessels with increased magnetic susceptibility values were detected surrounding the ischemic lesion at all times, indicating an increase in oxygen extraction. Immunohistochemistry revealed narrowed capillaries and dilated larger vessels. Thus, prominent vessels are an important indicator of underlying microvascular pathology and may by pivotal for diagnosis and therapeutic decision making in stroke patients.
Introduction
Prominent vessels in the brain of patients with ischemic stroke have been observed on susceptibility weighted images and quantitative susceptibility maps (QSM).1-3 Their occurrence has been attributed to an increase in oxygen extraction fraction and is correlated with misery perfusion in potentially salvable brain tissue.1,4 However, the occurrence of prominent vessels after restoration of reperfusion has so far not been evaluated. The goal of the current study was to perform QSM in the middle cerebral artery occlusion (MCAO) model of cerebral ischemia after several time points of reperfusion and to qualitatively and quantitatively assess the occurrence of prominent vessels. Moreover, immunohistochemistry was used to assess underlying vessel pathology in brain sections.Methods
Seventeen male C57BL6 mice underwent 1h of MCAO followed by reperfusion using the intraluminal filament technique.5 A Bruker PharmaScan 47/16 operating at 200MHz and equipped with a cryogenic transmit-receive coil was used for MRI. During acquisition mice were spontaneously breathing under isoflurane anesthesia (1.5%). A 3D multi-echo gradient recalled echo sequence was applied using a FOV=25.6 mm×25.6 mm×8 mm and an acquisition matrix=256×256×80, resulting in an effectively isotropic spatial resolution of 100 μm×100 μm×100 μm. Four echoes were recorded (TE1-4=4.5/10.5/16.5/22.5 ms) with TR=100 ms, flip angle=15°. Single-channel magnitude images were combined using the sum-of-squares method.6 Images for each echo were unwrapped using a 3D best-path algorithm.7 Background frequency contributions were eliminated using sophisticated harmonic artifact removal for phase data (SHARP), with 10 different spherical kernels with varying radii ranging from 100 µm to 1000 µm, employing a regularization parameter for truncated singular value decomposition of 0.05.8 Susceptibility maps were computed based on SHARP-processed frequency images using homogeneity enabled incremental dipole inversion (HEIDI).9 Brains were harvested, fixed and routinely paraffin wax embedded. Sections (3-5 µm) were prepared and incubated, with rabbit anti-mouse collagen IV for 15-18 h at 4°C. Subsequently, they were incubated with Envision rabbit, Dako.Results
Prominent vessels with high magnetic susceptibility were seen on magnetic susceptibility maps of the ischemic hemisphere on all time points (Figure 1a). They were mainly found ipsilateral in the territory supplied by the middle cerebral artery. On the contralateral hemisphere vessel-like structures were occasionally observed, but were only faintly visible against tissue background. Moreover, ipsilateral prominent vessels appeared larger in diameter than comparable vessels on the contralateral side. Furthermore, an increased number of prominent vessels were found in the ischemic hemisphere of mice imaged at 12h, 24h and 48h after reperfusion compared to mice imaged at 2h, 4h and 6h after reperfusion. VOI analysis revealed significantly higher differences in magnetic susceptibility (relative to CSF) at 2h and 4h after reperfusion in prominent vessels of the ischemic ipsilateral side compared to the contralateral hemisphere (Figure 1b). Immunohistological examination demonstrated that larger vessels appeared dilated compared to the contralateral side (Figure 1c) and capillaries showed swollen endothelial cells and narrowing of the vessel lumen (Figure 1d) in comparison to the capillaries in the contralateral hemisphere in the same location.Discussion
Previous studies described capillary constriction and impaired capillary reflow as a consequence of pericyte contraction.10,11 Thus, despite restoration of cerebral blood flow tissue oxygen availability is presumably lower compared to pre-ischemic values due to narrowed capillaries. The occurrence of prominent vessels in the surrounding area indicates a compensatory mechanism to maintain oxygen metabolism of tissue.Conclusion
Microvascular pathology hampers reperfusion of ischemic tissue and promotes secondary tissue injury. Thus, prominent vessels are an important indicator of underlying microvascular pathology and may by pivotal for diagnosis and therapeutic decision making in stroke patients.Acknowledgements
No acknowledgement found.References
- Luo Y, Gong Z, Zhou Y, et al. Increased susceptibility of asymmetrically prominent cortical veins correlates with misery perfusion in patients with occlusion of the middle cerebral artery. Eur Radiol. 2017;27:2381-2390.
- Chen CY, Chen CI, Tsai FY, et al. Prominent vessel sign on susceptibility-weighted imaging in acute stroke: prediction of infarct growth and clinical outcome. PLoS One 2015;10:e0131118.
- Kao HW, Tsai FY, Hasso AN. Predicting stroke evolution: comparison of susceptibility-weighted MR imaging with MR perfusion. Eur Radiol 2012;22:1397-403.
- Uwano I, Kudo K, Sato R, et al. Noninvasive assessment of oxygen extraction fraction in chronic ischemia using quantitative susceptibility mapping at 7 Tesla. Stroke 2017;48:2136-2141.
- Vaas M, Ni R, Rudin M, et al. Extracerebral tissue damage in the intraluminal filament mouse model of middle cerebral artery occlusion. Front Neurol 2017;8:85.
- Roemer PB, Edelstein WA, Hayes CE, et al. The NMR phased array. Magn Reson Med 1990;16:192-225.
- Abdul-Rahman HS, Gdeisat MA, Burton DR, et al. Fast and robust three-dimensional best path phase unwrapping algorithm. Appl Opt 2007;46:6623-6635.
- Schweser F, Deistung A, Lehr BW, et al. Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? Neuroimage 2011;54:2789-2807.
- Schweser F, Sommer K, Deistung A, et al. Quantitative susceptibility mapping for investigating subtle susceptibility variations in the human brain. Neuroimage 2012;62:2083-2100.
- Yemisci M, Gursoy-Ozdemir Y, Vural A, et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 2009;15:1031-1037.
- Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014;508:55-60.
Figures
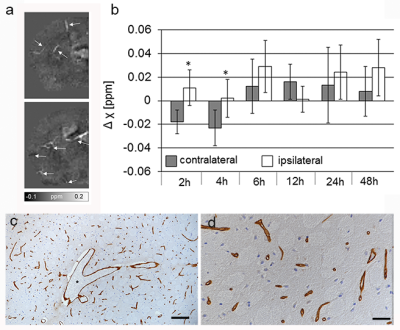
Figure 1 (a) Representative axial quantitative susceptibility maps of the
ischemic hemisphere of a mouse after MCAO and 6h of reperfusion. Prominent
vessels with higher magnetic susceptibilities are seen (white arrows). (b) Differences in magnetic
susceptibility in vessels on the ischemic ipsilateral and contralateral
hemisphere at different time points after 1h reperfusion. mean + SD. Mann-Whitney rank
sum test, *P < 0.05 compared
to contralateral side. (c, d) Anti-collagen IV
immunohistochemistry of the brain at 24h after reperfusion. (c) Larger vessels appear dilated (*).
Bar=100 µm. (d) Swelling of
endothelial cells and narrowing of the vessel lumen of capillaries. Bar=20 µm.