4798
Reduced cerebral blood flow and oxygen consumption in asymptomatic unilateral carotid stenosis patients assessed by arterial spin labeling and multi-parametric quantitative BOLD imaging1Department of Neuroradiology, Technische Universität München, Munich, Germany, 2Department of Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States, 3Clinic for Neurology, Technische Universität München, Munich, Germany
Synopsis
Assessing cerebral hemodynamics and oxygen consumption in patients with clinically asymptomatic, high-grade internal carotid artery stenosis is crucial to estimate risk of stroke and cognitive impairment. Here, 23 patients with unilateral high-grade carotid stenosis and 24 age-matched healthy controls underwent a range of MRI scans, including quantitative BOLD, DSC and pCASL imaging to assess relative oxygen extraction (OEF), blood flow (CBF) and oxygen consumption (CMRO2). Both CBF and CMRO2 were reduced on the stenosis side, indicating that the proposed easily applicable MR-protocol is useful to estimate even subtle CBF and CMRO2 changes in carotid stenosis patients.
Purpose:
Patients with high-grade internal carotid artery stenosis (ICAS) may exhibit cerebral hemodynamic impairment, being potentially reversible by surgical treatment.1 Assessing cerebral hemodynamics and oxygen metabolism is crucial in ICAS as stroke risk and cognitive functions depend on hemodynamics.2-4 MRI studies using calibrated BOLD imaging demonstrated changes in blood flow (CBF) and cerebral metabolic rate of oxygen (CMRO2) on the side of the stenosis,5 however, applicability in everyday clinical practice is fairly limited due to reduced tolerability of gas challenges in some patients. Here, we assessed the feasibility of a more tolerable MRI protocol in patients with asymptomatic, unilateral high-grade stenosis, including multi-parametric quantitative BOLD (mqBOLD), dynamic susceptibility contrast (DSC) and pseudo-continuous arterial spin labeling (pCASL), and compared respective hemodynamic parameters between cerebral territories of the affected and unaffected carotid arteries and to healthy controls.Methods:
23 patients (69.9±7.5y, 10 female) with one-sided extracranial, high-grade, clinically asymptomatic ICAS (>70%, according to the NASCET criteria) and 24 healthy, age-matched controls (70.1±5.0y, 15 female) were enrolled in this prospective study (Tab. 1). Participants underwent an MRI examination on a clinical 3T Ingenia scanner (Philips, Netherlands) using a 16-channel head-neck coil. The protocol included structural MRI (MP-RAGE and FLAIR) as well as perfusion MRI using pCASL (3D readout, individual labeling by PCA-survey at least 2cm above the stenosis, label duration 1800ms, post-label delay 2000ms, background suppression with four pulses, voxel size 2.73x2.86x6mm3, 16 slices, 3 dynamics)6. A labeling efficiency of 0.75 was assumed.7,8 Arterial transit time artefacts were excluded via visual inspection (by JG) and analysis of the spatial coefficient of variation in each native hemisphere.9 Furthermore, we conducted motion-corrected mqBOLD imaging with T2*-mapping (multi-echo-GRE, TEmin=∆TE=5ms, TR=1950ms, α=30°, 12 echos, voxel size 2x2x3mm3, 30 slices) and T2-mapping (multi-echo-GraSE, TEmin=∆TE=16ms, TR=8971ms, 8 echos, voxel size 2x2x3mm3, 30 slices)10 to obtain R2'($$$=\frac{1}{T2^*}- \frac{1}{T2}$$$). Relative cerebral blood volume (rCBV) maps were generated based on a DSC scan (TE=30ms, TR=1500ms, α=60°, voxel size 2x2x3mm3, 80 dynamics, weight adjusted 15-20ml Gd-DOTA, flow rate 4ml/s)11. The relative oxygen extraction fraction (rOEF) was calculated as $$rOEF=\frac{R2'}{c\cdot rCBV}$$ with $$$c=\frac{4}{3}\cdot \gamma\cdot \pi\cdot \Delta\chi\cdot B_0$$$ (see 10). Relative CMRO2 maps were calculated according to $$$rCMRO_2=C_a\cdot rOEF\cdot CBF$$$ with Ca being the arterial oxygen content (19ml/(100ml blood))12. All parameter maps were normalized to MNI space using SPM12 and mean values were extracted within each hemisphere’s anterior circulation using vascular territory maps of the anterior and middle cerebral arteries13 (Fig. 1A). We excluded frontobasal and temporopolar regions because of susceptibility artefacts especially in T2* (weighted) images (i.e. Brodmann’s areas (BA) 11, 20, 25, 30, 34, 36, 38) and the basal ganglia due to known iron deposits in these regions (BA 47-55). To validate pCASL derived absolute CBF, we compared values from nine young healthy subjects (mean age 25.8±3.2y, 4 female) to CBF values assessed by 15O-H20 PET in 13 young male subjects (26.1±3.8y) from another study14 across BAs (Fig. 1B). For comparisons between groups and hemispheres, we used two-sample and paired t-tests, respectively, with a significance level of p<0.05.Results:
In young healthy subjects, CBF derived from pCASL and PET showed good agreement across BAs, except for the visual cortex (BA 17), where pCASL yielded lower values (two sample t-test: p=0.011, Fig. 1B). Absolute values of R2', rCBV, rOEF, CBF and rCMRO2 did not differ between patients and elderly controls. However, patients showed significantly reduced CBF and rCMRO2 values in the cerebral territory of the stenosed carotid artery compared to the contralateral territory (paired t-test: each p<0.001; Fig. 2, 3, 4). rOEF values did not differ between the affected and unaffected side (Fig. 3, 4). Spatial coefficient of variation of CBF was not significantly increased on the side of the stenosis (mean±SD affected/unaffected side: 38.3±5.2/37.6±7.0, paired t-test: p=0.583).Discussion:
In patients with high-grade unilateral carotid stenosis, we revealed relatively reduced CBF and rCMRO2 in the cerebral territories of the stenosed carotid arteries using mqBOLD, DSC and pCASL. R2', rCBV, and rOEF did not show any differences between the affected and unaffected sides, being in line with previous studies using calibrated BOLD5 and PET15. The unchanged spatial coefficient of variation of native unprocessed CBF data suggests that detected CBF reductions are not resulting from an increased arterial transit time.9 rCMRO2 reductions are primarily driven by reduced CBF.Conclusion:
These results indicate that subtle ipsilateral perfusion and oxygen metabolism reductions due to one-sided high-grade carotid stenosis can be detected by an easily applicable MR-protocol including mqBOLD, DSC, and pCASL. Whether these potentially treatable chronic hemodynamic changes lead to structural or cognitive impairments needs to be elucidated in future studies.Acknowledgements
The authors would like to thank the Faculty of Medicine of the Technische Universität München and the Dr.-Ing. Leonhard Lorenz-Stiftung for supporting this work. We also thank Philips Healthcare, Best, the Netherlands, for helping with pCASL sequence implementation.References
1. Kawai N, Hatakeyama T, Okauchi M, et al. Cerebral blood flow and oxygen metabolism measurements using positron emission tomography on the first day after carotid artery stenting. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association 2014;23:e55-64.
2. Silvestrini M, Paolino I, Vernieri F, et al. Cerebral hemodynamics and cognitive performance in patients with asymptomatic carotid stenosis. Neurology 2009;72:1062-8.
3. Silvestrini M, Vernieri F, Pasqualetti P, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA : the journal of the American Medical Association 2000;283:2122-7.
4. Marshall RS. Effects of altered cerebral hemodynamics on cognitive function. Journal of Alzheimer's disease : JAD 2012;32:633-42.
5. De Vis JB, Petersen ET, Bhogal A, et al. Calibrated MRI to evaluate cerebral hemodynamics in patients with an internal carotid artery occlusion. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2015;35:1015-23.
6. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2015;73:102-16.
7. Mutsaerts HJ, Steketee RM, Heijtel DF, et al. Inter-vendor reproducibility of pseudo-continuous arterial spin labeling at 3 Tesla. PloS one 2014;9:e104108.
8. Garcia DM, Duhamel G, Alsop DC. Efficiency of inversion pulses for background suppressed arterial spin labeling. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2005;54:366-72.
9. Mutsaerts HJ, Petr J, Vaclavu L, et al. The spatial coefficient of variation in arterial spin labeling cerebral blood flow images. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2017:271678X16683690.
10. Hirsch NM, Toth V, Forschler A, et al. Technical considerations on the validity of blood oxygenation level-dependent-based MR assessment of vascular deoxygenation. NMR in biomedicine 2014;27:853-62.
11. Kluge A, Lukas M, Toth V, et al. Analysis of three leakage-correction methods for DSC-based measurement of relative cerebral blood volume with respect to heterogeneity in human gliomas. Magnetic resonance imaging 2016;34:410-21.
12. An H, Lin W, Celik A, et al. Quantitative measurements of cerebral metabolic rate of oxygen utilization using MRI: a volunteer study. NMR in biomedicine 2001;14:441-7.
13. Tatu L, Moulin T, Vuillier F, et al. Arterial territories of the human brain. Frontiers of neurology and neuroscience 2012;30:99-110.
14. Hyder F, Herman P, Bailey CJ, et al. Uniform distributions of glucose oxidation and oxygen extraction in gray matter of normal human brain: No evidence of regional differences of aerobic glycolysis. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2016;36:903-16.
15. Bokkers RP, Bremmer JP, van Berckel BN, et al. Arterial spin labeling perfusion MRI at multiple delay times: a correlative study with H(2)(15)O positron emission tomography in patients with symptomatic carotid artery occlusion. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2010;30:222-9.
16. Zhang K, Herzog H, Mauler J, et al. Comparison of cerebral blood flow acquired by simultaneous [15O]water positron emission tomography and arterial spin labeling magnetic resonance imaging. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2014;34:1373-80.
Figures
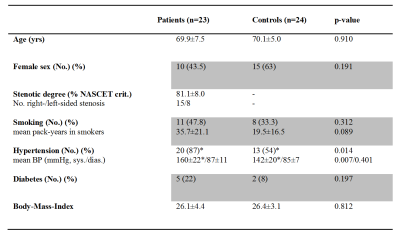
Table 1: Clinical characteristics
p-values of two-sample t-tests (age, stenotic degree, mean blood pressure (BP), and Body-Mass-Index) and chi-squared tests (sex, number of smoking, hypertensive, and diabetic participants)
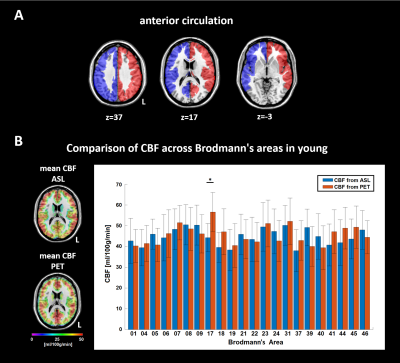
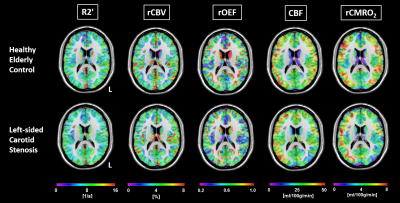
Figure 2. Example of multi-parametric imaging in a control and a carotid stenosis patient
Parameter maps of R2', rCBV, rOEF, CBF and rCMRO2 in a healthy elderly control (top row) and a left-sided carotid stenosis patient (bottom row) within gray matter in MNI space. Notice reductions of CBF and rCMRO2 in the patient on the side of the stenosis, whereas R2', rCBV and the resulting rOEF maps are symmetrical.
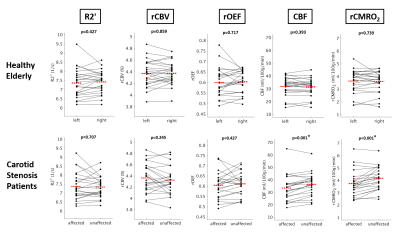
Figure 3. Comparison of the anterior circulation of both hemispheres in healthy controls and patients
Paired scatter plots depicting mean R2', rCBV, rOEF, CBF and rCMRO2 values between the left vs. right, and affected (=ipsilateral to the stenosis) vs. unaffected (=contralateral) side of the anterior circulation in controls and patients, respectively. Significant intra-subject side differences have been observed only for CBF and rCMRO2 in patients (paired t-test, p<0.001 for both parameters). The red dashed line indicates the mean parameter values of the respective side.
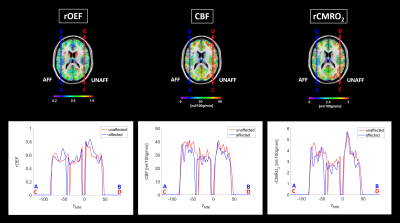
Figure 4. Region-of-interest analysis of the affected vs. unaffected hemisphere in patients
Patients’ rOEF, CBF and rCMRO2 maps were flipped along the mid-sagittal axis so that all hemispheres ipsilateral to the stenosis were set to the right side (AFF). Afterwards, normalization to MNI space and calculation of averaged parameter maps was conducted (top row). Exemplary profiles of affected and unaffected hemispheres were created within an axial slice at MNI coordinate zMNI=17 along the y-direction (yMNI) at xMNI=-48 (affected side, blue lines and corresponding plots from A to B) and xMNI=48 (unaffected side, red lines and corresponding plots from C to D).