4797
Improving Cerebrovascular Reactivity Assessment Using High-Resolution MB-EPI Multi-Delay PCASL Imaging1Center for Magnetic Resonance Research, School of Medicine, University of Minnesota, Minneapolis, MN, United States, 2Laboratory of Integrative Human Physiology, School of Kinesiology, University of Minnesota, Minneapolis, MN, United States, 3Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, MN, United States, 4Neuropsychology Section, Hennepin County Medical Center, Minneapolis, MN, United States, 5Berman Center for Clinical Research, Hennepin County Medical Center, Minneapolis, MN, United States
Synopsis
Arterial spin labeling (ASL) imaging with a respiratory challenge can provide both quantitative baseline cerebral blood flow and the assessment of the cerebrovascular reactivity (CVR), an index for cerebrovascular function. However, to date, low-resolution and single-delay ASL imaging is primarily applied to assess CVR, and therefore limited. We proposed and successfully applied high-resolution multi-delay pseudo-continuous ASL (PCASL) imaging using a slice accelerated EPI readout for respiratory challenge studies. The study results suggest that the respiratory challenge can induce significant changes in arterial transit time (ATT), and that the estimates of ATT from the multi-delay imaging protocol are critical to achieve unbiased CVR measurements.
Purpose
Decline in cerebrovascular function can play an essential role in the initiation and progression of cerebrovascular diseases and cognitive impairment 1. Cerebrovascular function can be assessed via evaluating cerebrovascular reactivity (CVR) by performing blood oxygen level dependent (BOLD) 2, T2* mapping 3, or arterial spin labeling (ASL) imaging 4 with a respiratory challenge. Compared to other imaging methods, ASL imaging with a respiratory challenge can provide both baseline cerebral blood flow (CBF) and CVR, and involves the manipulation of end-tidal partial pressure of CO2 (PetCO2) and O2 (PetO2) to challenge blood vessels of the brain. To date, low-resolution and single-delay ASL imaging has been primarily applied to the evaluation of CVR. This limits the ability to achieve robust CVR measurements in thin or atrophic cortex, as well as in small subcortical structures, and will result in systematic biases for CBF estimates (particularly for CVR measures) because the changes of arterial transit time (ATT) following a respiratory challenge are not accounted for. To overcome these limitations, we proposed and applied high-resolution multi-delay pseudo-continuous arterial spin labeling (PCASL) imaging using a multi-band (MB) EPI readout 5 to measure CVR. The purpose was to evaluate the feasibility and benefits of the proposed imaging approach for future clinical research.Methods
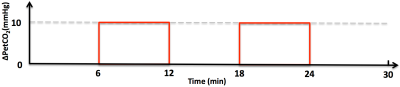
Studies with healthy volunteers were performed on a Siemens 3T MRI scanner using a 32-channel head coil under an IRB approved protocol with written informed consent. A computer-controlled gas-blending device was used to evaluate baseline and manipulate end-tidal carbon dioxide (PetCO2). A prospective PetCO2 targeting approach was employed to produce PetCO2 values to within ±1 mmHg and constrained PetO2 (< 10 mmHg) 6-9. The targeted PetCO2 challenge was achieved by increasing the level of PetCO2 10 mmHg above each subject’s baseline while PetO2 was held constant throughout the study as shown in Figure 1.
The major parameters for high-resolution MB-EPI multi-delay PCASL imaging are as follows: in-plane resolution = 2.5 x 2.5 mm3, slice thickness/gap = 2.27 mm/10%; number of slices = 60; labeling duration = 1.5 s; five post-bolus delays (PLDs) = {0.2, 0.7, 1.2, 1.7, 2.2} s, # of measurements for five PLDs = {12, 12, 12, 20, 30}; MB-EPI factor = 6, and total acquisition time = ~ 5 mins. A series of noise images along with two fully relaxed M0 images were obtained separately. PCASL imaging acquisition was started 20 s after the changes of respiratory conditions to avoid potential confounding effects from the transition of respiratory conditions.
Post-imaging processing was performed with the FSL toolbox and SPM software. The single-blood compartment model was used for CBF quantification 10. CBF quantification for multi-delay PCASL imaging was achieved by using Oxford’s ASL tool in the FSL toolbox 11; CBF quantification for single-delay PCASL imaging data was obtained using in-house MATLAB scripts 5. The CVR was evaluated as the PetCO2-induced percent changes of CBF within both the grey and white matter.
Results and Discussion
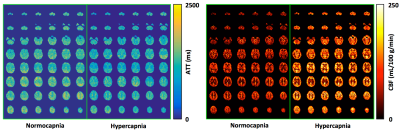
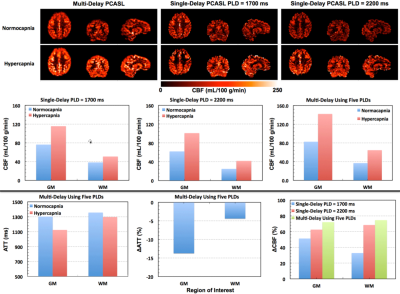
To our knowledge, this is the first time that whole-brain CVR measurement has been achieved at high-resolution (nominal isotropic 2.5 mm3) using a multi-delay PCASL imaging protocol. High quality ATT and CBF maps under normocapnia and hypercapnia conditions were successfully obtained from all subjects by using a single corresponding multi-delay data set acquired within about 5 mins, which confirms that the respiratory challenge paradigm (Figure 1) can be simplified to have one normocapnia condition and one hypercapnia condition, making the duration of the respiratory challenge study protocol more amenable to clinical research.
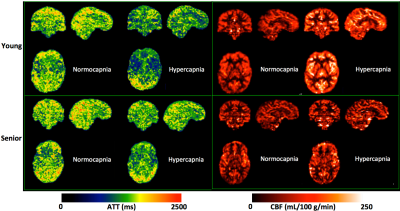
These results also suggest that the targeted PetCO2 challenge significantly reduces ATT (Figures 2-4). If a single-delay PCASL imaging acquisition is employed without accounting for ATT differences between conditions, the CVR can be greatly underestimated, especially when a typical short PLD (e.g., 1.7 s) is used (Figure 3). Furthermore, the magnitude of ATT alterations induced by the respiratory challenge may change with aging (Figure 4) or cerebrovascular diseases, and is a valuable physiological parameter complementary to the estimated CVR. Research questions raised by these results, such as how the multi-delay imaging study approach affects the sensitivity of measured CVR to aging, are being investigated.
Conclusions
The proposed high-resolution multi-delay PCASL imaging protocol was successfully applied to respiratory challenge studies. The estimates of ATT are critical to achieve unbiased CVR measurements.Acknowledgements
P41 EB015894, P30 NS076408 and UL1TR000114. This research work is also supported by the University of Minnesota Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.References
1. Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15(8):1983-1992.
2. Vesely A, Sasano H, Volgyesi G, et al. MRI mapping of cerebrovascular reactivity using square wave changes in end-tidal PCO2. Magn Reson Med. 2001;45(6):1011-1013.
3. Rossi C, Boss A, Donati OF, et al. Manipulation of cortical gray matter oxygenation by hyperoxic respiratory challenge: field dependence of R(2) * and MR signal response. NMR Biomed. 2012;25(8):1007-1014.
4. Tancredi FB, Gauthier CJ, Madjar C, et al. Comparison of pulsed and pseudocontinuous arterial spin-labeling for measuring CO2 -induced cerebrovascular reactivity. J Magn Reson Imaging. 2012;36(2):312-321.
5. Li X, Wang D, Auerbach EJ, Moeller S, Ugurbil K, Metzger GJ. Theoretical and experimental evaluation of multi-band EPI for high-resolution whole brain pCASL Imaging. Neuroimage. 2015;106:170-181.
6. Mark CI, Slessarev M, Ito S, Han J, Fisher JA, Pike GB. Precise control of end-tidal carbon dioxide and oxygen improves BOLD and ASL cerebrovascular reactivity measures. Magn Reson Med. 2010;64(3):749-756.
7. Prisman E, Slessarev M, Han J, et al. Comparison of the effects of independently-controlled end-tidal PCO(2) and PO(2) on blood oxygen level-dependent (BOLD) MRI. J Magn Reson Imaging. 2008;27(1):185-191.
8. Slessarev M, Han J, Mardimae A, et al. Prospective targeting and control of end-tidal CO2 and O2 concentrations. J Physiol. 2007;581(Pt 3):1207-1219.
9. Dengel DR, Evanoff NG, Marlatt KL, Geijer JR, Mueller BA, Lim KO. Reproducibility of blood oxygen level-dependent signal changes with end-tidal carbon dioxide alterations. Clin Physiol Funct Imaging. 2017;37(6):794-798.
10. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73(1):102-116.
11. Chappell MA, MacIntosh BJ, Donahue MJ, Gunther M, Jezzard P, Woolrich MW. Separation of macrovascular signal in multi-inversion time arterial spin labelling MRI. Magn Reson Med. 2010;63(5):1357-1365.
Figures