4795
Rigid co-registration for MOLLI, a clinical test.1Radiology Department, Beijing Hospital, Beijing, China, 2Philips Healthcare, Beijing, China
Synopsis
T1 quantification requires acquisition of multiple images at a sequence of delay times to derive the T1 recovery curve. Modified look-locker inversion recovery (MOLLI) is one of the most commonly used techniques. However, motion between these image acquisitions, especially at different heartbeats, often results in artifacts. In this work, we evaluated motion correction via a novel rigid co-registration based on images at a series time of delay along the T1 relaxation to eliminate the respiratory and cardiac motion artifacts.
Synopsis
T1 quantification requires acquisition of multiple images at a sequence of delay times to derive the T1 recovery curve. Modified look-locker inversion recovery (MOLLI) is one of the most commonly used techniques. However, motion between these image acquisitions, especially at different heartbeats, often results in artifacts. In this work, we evaluated motion correction via a novel rigid co-registration based on images at a series time of delay along the T1 relaxation to eliminate the respiratory and cardiac motion artifacts.Purpose
Myocardial T1 mapping sequences enable quantitative assessment of myocardial fibrosis in many cardiomyopathies and have shown a broad development perspective in some studies1. In these sequences, T1 quantification requires obtaining a series of images at varying delay times to derive the MR longitudinal relaxation T12. In modified look-locker inversion recovery (MOLLI), a set of IR experiments with increasing Tis in each IR are performed throughout one breath hold. Although myocardial T1 mapping sequences usually acquire images within breath-hold conditions, motion between these images still result in artifacts. The mismatch between the later delay time images and the initial ones causes substantial errors in the calculated maps3. Previously non-rigid image registration for cardiac T1 mapping has been developed, but its stability is still challenged in patient applications. Recently a rigid image registration for each IR with windowing the FOV size to zoom and verify the results was developed. Hence, this rigid registration method assumed good repetition of heart shape during the heart beat during image acquisition. Herein we investigate if such assumption and associated image reregistration could improve T1 mapping in MOLLI sequence and eliminate the respiratory and cardiac motion artifacts.Methods
Clinical images from 14 patients (8 hypertrophic cardiomyopathy, 2 myocardial infarction, 3 dilated cardiomyopathy and 1 myocardial metastasis from lung cancer) with MOLLI scan examined were collected retrospectively in this study; among which 12 patients underwent contrast administration. All imaging was performed using a 3.0T multi-transmit magnetic resonance scanner (Achieva 3.0T TX, Philips, Best, the Netherlands). MOLLI acquisitions were performed before and after 0.2mmol/kg gadolinium based contrast agent (GBCA) administration. The schemes for native and post contrast (enhanced) acquisition are 5s(3s)3s and 4s(1s)3s(1s)2s; and a total of 8 and 9 images were obtained respectively. Images were acquired at 3 short-axis slices: apical, mid-cavity and basal part for each subject. These T1 mapping sequences were applied with single hold for each slice, end-expiratory, electrocardiography-gated for image acquisition, and bSSFP readout (TR/TE=2.1ms/0.95ms, flip angle=20°, FOV=300×300mm2, voxel size=2.0×2.0mm2, slice thickness=10mm, SENSE acceleration factor=2, minimum inversion time: 110 ms, inversion-time increment: 80 ms). Quantitative T1 maps for native and post contrast were calculated based on the reordered images at each delay time from each repetitions. Further post-processing for image co-registration was applied using the tool under developing in Intellespace Portal (Philips, Best, Netherland). FOV sizes were windowed to fit myocardium in space; and rigid registration was applied for T1 quantification. We categorized all the T1 maps into poor, fair and good on the basis of image quality and T1 recovery curve; and compared the T1 maps before and after the proposed correction to assess the image co-registration tool’s feasibility in cardiac T1 mapping.Results
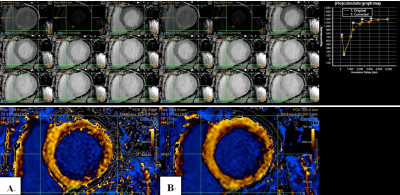
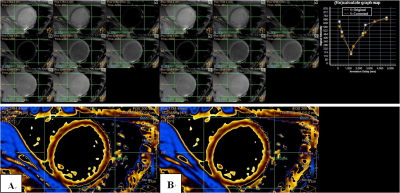
Among the native 42 T1 maps, 14 (33.3%) T1 maps were improved after the rigid registration motion correction; and 20 (55.6%) T1 maps improved among the post contrast 36 T1 maps. Although most T1 maps before motion co-registration were already excellently acceptable for clinical use, we could still find post-processed signals at different TI times better fit the T1 recovery curve after co-registration and result in more accurate T1 values. Among all the cases, edge of heart is the most sensitive part to motion artifact. Thus, we put the ROI in this area to challenge the algorithm and evaluate it. Figure 1 shows motion correction removes the artifacts associated with motion between different TI images. Besides, the calculate graph map shows the corrected T1 recovery curve gets better than the original one. Figure 2 shows T1 maps without any visible artifacts may also benefit from the proposed reconstruction approach. The better-fit T1 recovery curves usually generate more accurate T1 estimations.Conclusion
This investigation confirmed that this rigid image registration for correcting motion artifacts in T1 mapping image did improve image quality of T1 maps, and improve the accuracy of T1 maps in the absence of motion artifacts. A good repetition of heart shape during the heart beat during image acquisition was the assumption in applying rigid registration. More image cases are to be recruited to further investigate the limit of this T1 mapping reregistration method.Acknowledgements
This work was supported by the National Key Clinical Specialty Construction Project of China.References
1. Dass S, Suttie JJ, Piechnik SK, et al. Myocardial tissue characterization using magnetic resonance noncontrast T1 mapping in hypertrophic and dilated cardiomyopathy. Circulation Cardiovascular imaging. 2012;5(6):726-733.
2. Burt JR, Zimmerman SL, Kamel IR, Halushka M, Bluemke DA. Myocardial T1 mapping: techniques and potential applications. Radiographics. 2014;34(2):377-395.
3. Moon JC, Messroghli DR, Kellman P, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. Journal of Cardiovascular Magnetic Resonance. 2013;15:92.
4. Slomka PJ, Baum RP. Multimodality image registration with software: state-of-the-art. European journal of nuclear medicine and molecular imaging. 2009;36 Suppl 1:S44-55.
Figures