4794
4D Segmentation of Whole-heart Cine Cardiovascular Magnetic Resonance Imaging in Congenital Heart Disease1Health Sciences & Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 2Electrical Engineering & Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 3Boston Children’s Hospital and Harvard Medical School, Boston, MA, United States
Synopsis
3D cine whole-heart cardiovascular magnetic resonance (MR) imaging promises to enable more accurate quantitative evaluation of cardiac function. To extract volumetric measurements of heart chambers and vascular structures from 3D cine datasets, we utilize a graph cuts segmentation algorithm and a deformable registration method. Our measurements differ from those acquired from the conventional 2D cine images, suggesting measurements of ventricular volume based on 2D cine lack accuracy. Clinical evaluation of heart function can therefore be improved by using 3D cine cardiovascular MR imaging coupled with automatic whole heart segmentation.
Purpose
Quantitative evaluation of cardiac function from magnetic resonance (MR) images promises to greatly improve the assessment of patients with congenital heart defects. In current clinical practice, a stack of 10-12 2D cine cardiovascular MR images acquired during breathhold are manually segmented to estimate left and right ventricular volumes at end systolic and end diastolic cardiac phases only. The accuracy of this approach is limited by slice-to-slice misalignment across the 2D image stacks and poor through-plane resolution. Furthermore, the current practice of capturing just two time points in the cardiac cycle precludes quantitative evaluation of cardiac motion over the entire cardiac cycle. As an alternative, free-breathing 3D cine cardiovascular MR imaging gated to the respiratory cycle provides both high in-plane and through-plane resolution and removes the potential for breathhold related slice misregistration, promising more accurate and robust volume measurements not only of the heart chambers, but also of the vascular structures (such as the aorta, pulmonary arteries/veins, superior/inferior vena cava) throughout the cardiac cycle. Manual segmentation of 3D volumes at multiple time points in the cardiac cycle is impractical since it requires 4-8 hours of work per volume. To enable applications in a clinical setting, we propose a robust algorithm to segment the entire volumetric series automatically. Our preliminary approach depends on a segmentation of one volume in the series; future work will aim to eliminate this requirement.Materials and Methods
Imaging: To assess the feasibility of the proposed segmentation algorithm, four patients (three male, median age: 18 years) with congenital heart defects were provided informed consent and imaged with the free-breathing, respiratory-gated 3D cine cardiovascular MR sequence2 after the administration of 0.15 mmol/kg gadobutral contrast. The imaging was performed on a 1.5T Philips MR scanner with the following parameters: field-of-view ≈512 (SI) × 250 (AP) × 180 (RL) mm, isotropic spatial resolution of 2.0mm, 30 heart phases, flip angle of 60°, TE/TR=1.52/3.0 ms, bandwidth ≈1.74 kHz, respiratory acceptance window of 3 mm with a tracking factor of 1 and 40% gating efficiency, a 28-element phased-array coil, and SENSE x3. The clinical breathhold 2D cardiovascular MR sequence was acquired with the following imaging parameters: field-of-view ≈270 (SI) × 270 (RL) × 107 (AP) mm, spatial resolution of 1.8 × 1.8 × 8 mm; slice gap of 1, 2 heart phases, flip angle of 60°, TE/TR=1.4/2.8 ms, bandwidth ≈1.08 kHz, and SENSE x2. Analysis: The 3D cine cardiovascular MR images are segmented as follows. First, a coarse manual segmentation is provided for a 3D volume at a reference volume in the cardiac cycle (end-diastole in this study). We employ a graph cuts segmentation algorithm3 to refine the coarse manual segmentation. Next, we apply diffeomorphic registration4 to align the remaining 29 volumes in the series to the segmented reference volume, and propagate the segmentation label maps to each volume in the series. We refine the propagated segmentations using the same graph cuts algorithm3 to account for residual misalignments. We smooth the final segmentations and remove isolated island pixels. We emphasize that the proposed method provides segmentation not only of the ventricles and atria, but also of the great vessels.Results
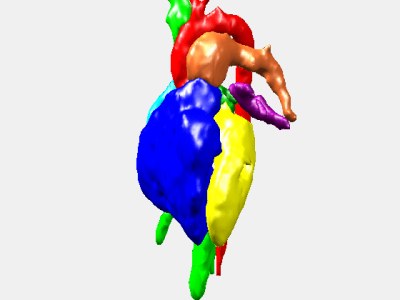
Figure 1 reports the left and right ventricular volumes for end-diastolic and end-systolic cardiac phases for the 3D and 2D cine cardiovascular MR images. In particular, we note that the volume measurement based on the 2D MR images produces underestimation of the right ventricular volumes and overestimation of the left ventricular volumes when compared to our measurements in 3D cine MR series (summarized in Table 1). Figure 2 illustrates two representative coronal slices of the segmented 3D cine MR image. Figure 3 presents a 3D rendering of the segmented 3D cine images throughout the cardiac cycle.Conclusions
We propose and demonstrate a method to segment cardiac chambers and great vessels from 3D cine cardiovascular MR images, enabling detailed and accurate measurements of clinically important heart chamber volumes. We observe differences between the ventricular volumes measured by our technique and those estimated from the clinical 2D cine MR images. These differences could be in part due to different breathing pattern (i.e., breath-hold vs. free-breathing) and poor through-plane resolution of 2D image stacks.Acknowledgements
NIH NIBIB NAC P41EB015902, NIH NICHD U01HD087211, Wistron Corporation, Philips.References
1. Grothues F, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. American Journal of Cardiology 2002; 90:29–34.
2. Moghari MH, et al. Free-breathing whole-heart 3D cine magnetic resonance imaging with prospective respiratory motion compensation. Magnetic Resonance Imaging 2017 (in production); DOI: 10.1002/mrm.27021.
3. Tang M, et al. GrabCut in one cut. IEEE International Conference on Computer Vision and Pattern Recognition 2013.
4. Avants, B, et al. Symmetric diffeomorphic image registration with cross correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis 2008; 12:26-41.
Figures