4788
Augmenting the interpretation of cardiac MRI by biomechanical modeling: Application to tetralogy of Fallot1Inria, Paris-Saclay University, Palaiseau, France, 2LMS, Ecole Polytechnique, Paris-Saclay University, Palaiseau, France, 3School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 4Department of Pediatrics, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
We propose connecting the acquisition and processing of cardiovascular MR (CMR) data with biomechanical cardiac modeling. Physical and physiological character predisposes biomechanical models to predictivity, and CMR data turn them into patient-specific regime. A high computational demand is addressed by applying a spatially-reduced model: the geometry and kinematics are simplified, but all other physical properties kept. The approach is illustrated by example of Tetralogy of Fallot patients, in whom we are able to access the myocardial contractility pre- and post-pulmonary valve replacement aiming at deciding on optimal therapy timing – the problem that has not been completely solved by sole CMR.
Introduction
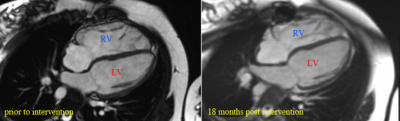
Patients with repaired tetralogy of Fallot (rToF) suffer from right ventricular (RV) volume and/or pressure overload due to chronic pulmonary regurgitation (and possible residual RV outflow tract stenosis) leading to RV dilatation (hypertrophy). Pulmonary valve replacement (PVR) is an intervention of choice. Timely PVR may cease the pathological RV remodeling, and the ventricle may even reverse-remodel back to normal size (Fig. 1). Considering the lifespan of a biological valve being ~10 years1, however, PVR tends to be deferred ideally until the latest point before irreversible changes of RV occur.
Cardiovascular Magnetic Resonance (CMR) is an invaluable technique in assessing the morphological and functional properties and the progress of remodeling changes during chronic ventricular overloading thanks to a limited inter-observer variability2 and no ionizing radiation. Among the main CMR indicators for performing PVR belong RV EDV, ESV, level of valvular regurgitation and RV ejection fraction. These measures, however, do not provide a sufficient sensitivity and specificity3 to predict the RV reverse-remodeling, therefore other criteria need to be sought. In this work, we suggest including biomechanical modeling4 – which puts into consideration physical and physiological principles of the cardiovascular system – to augment the information obtained from the acquired and processed CMR. By constraining the biomechanical model by clinical data, we access additional information not directly visible in the data, e.g. the parameters of myocardial contractility, relevant for the ventricular reverse-remodeling.
Methods
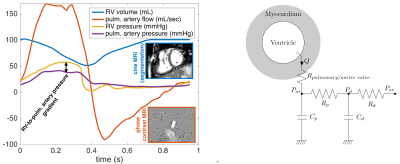
Cine MRI covering the heart ventricles in short axis, and the phase-contrast flow through the aortic and pulmonary valves were acquired prior to percutaneous PVR of 3 rToF patients. Additionally, one healthy subject with CMR and invasive ventricular pressures was included. MRI data were processed to time-vs-ventricular volume, and time-vs-flow by using image registration-based segmentation (IRTK library5 included in the visualizer Eidolon6). Pressures in the RV, pulmonary artery, LV and aorta were recorded during percutaneous PVR before and after deploying the valve. The volume, flow and pressure data were combined into a common time-space (Fig. 2).
The spatially-reduced order biomechanical model7 of a single ventricle (geometry and kinematics are simplified, but all other physical properties correspond to the complex 3D heart model8, Fig. 2) was calibrated separately to the LV and RV of each patient by using the measured data and following the steps:
- The Windkessel models representing pulmonary and systemic circulations (for RV, and LV, respectively), namely the proximal and distal resistances and capacitances Rp, Cp, Rd, Cd were identified by imposing the measured effective flow to the circulation for Rd, Cd, and the forward through-valve flow for Rp, Cp.
- The size of the spherical geometry and wall thickness were adjusted to the patient’s ventricular volume and myocardial mass extracted from cine MRI.
- The passive properties of the model (myocardial stiffness, represented by Holzapfel-Ogden model9) were adjusted by using the experimental data10, while imposing the measured end-diastolic intraventricular pressure (preload).
- The resistance of the pulmonary valve was adjusted to obtain the RV-to-pulmonary artery pressure gradient as in the data. Active properties of the model (myocardial contractility) were adjusted according to the measured stroke volume.
- Active properties of the model (myocardial contractility) were adjusted according to the measured stroke volume.
Results
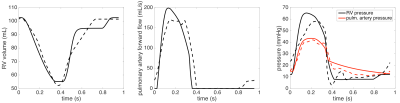
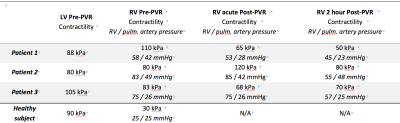
Fig. 3 demonstrates that our spatially-reduced model can be calibrated to the patient’s data even for the pathological RV. The values of myocardial contractilities for each ventricle (in all subjects) are displayed in Table 1. Firstly, in comparison to the healthy RV, the contractility in all rToF cases is chronically increased as the RV works against an increased afterload. Thanks to the model, we can also quantify a reduction of RV contractility post-intervention: a nearly complete normalization in Patient 1, a decrease by 15-20% in Patient 3 (clearly beneficial for the patients), however, no reduction in Patient 2.Conclusion
Turning a 3D model8 into patient-specific regime provides some clinically relevant predictive capabilities11. Due to a high computational demand and complexity of setting up the model, this time-consuming process should be rather seen as an offline platform after CMR exam. The spatially-reduced order model used in this work, however, is fast to set up, can run nearly real-time, and provides an additional insight into the acquired MRI data. This preliminary work shows potential in coupling simplified biomechanical modeling techniques with the MR imaging process. A direct inclusion within the MRI console could be realistically envisioned to augment the interpretation of the CMR exam. This study used interventional pressures in addition to CMR (XMR procedure). The potential of the model while using the invasive data, and also solely non-invasive CMR will be further explored to predict the long-term reverse remodeling12.Acknowledgements
No acknowledgement found.References
1. Oosterhof T, Meijboom FJ, Vliegen HW, et al. Long-term follow-up of homograft function after pulmonary valve replacement in patients with tetralogy of Fallot. Eur Heart J. 2006;27(12):1478-1484. doi:10.1093/eurheartj/ehl033.
2. Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004;147(2):218-223. doi:10.1016/j.ahj.2003.10.005.
3. Quail MA, Frigiola A, Giardini A, et al. Impact of pulmonary valve replacement in tetralogy of fallot with pulmonary regurgitation: A comparison of intervention and nonintervention. Ann Thorac Surg. 2012;94(5):1619-1626. doi:10.1016/j.athoracsur.2012.06.062.
4. Chabiniok R, Wang VY, Hadjicharalambous M, et al. Multiphysics and multiscale modelling, data–model fusion and integration of organ physiology in the clinic: ventricular cardiac mechanics. Interface Focus. 2016;6(2):20150083. doi:10.1098/rsfs.2015.0083.
5. Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. 1999;18(8):712-721.
6. Kerfoot E, Fovargue L, Rivolo S, et al. Eidolon: Visualization and Computational Framework for Multi-modal Biomedical Data Analysis BT - Medical Imaging and Augmented Reality: 7th International Conference, MIAR 2016, Bern, Switzerland, August 24-26, 2016, Proceedings. In: Zheng G, Liao H, Jannin P, Cattin P, Lee S-L, eds. Cham: Springer International Publishing; 2016:425-437. doi:10.1007/978-3-319-43775-0_39.
7. Caruel M, Chabiniok R, Moireau P, Lecarpentier Y, Chapelle D. Dimensional reductions of a cardiac model for effective validation and calibration. Biomech Model Mechanobiol. 2014;13(4):897-914. doi:10.1007/s10237-013-0544-6.
8. Chapelle D, Le Tallec P, Moireau P, Sorine M. Energy-Preserving Muscle Tissue Model: Formulation and Compatible Discretizations. Int J Multiscale Comput Eng. 2012;10(2):189-211. doi:10.1615/IntJMultCompEng.2011002360.
9. Holzapfel GA, Ogden RW. Constitutive modelling of passive myocardium: a structurally based framework for material characterization. Philos Trans R Soc A Math Phys Eng Sci. 2009;367(1902):3445-3475. doi:10.1098/rsta.2009.0091.
10. Klotz S, Hay I, Dickstein ML, et al. Single-beat estimation of end-diastolic pressure-volume relationship: a novel method with potential for noninvasive application. Am J Physiol Heart Circ Physiol. 2006;291(1):403-412. doi:10.1152/ajpheart.01240.2005.
11. Sermesant M, Chabiniok R, Chinchapatnam P, et al. Patient-specific electromechanical models of the heart for the prediction of pacing acute effects in CRT: a preliminary clinical validation. Med Image Anal. 2012;16(1):201-215. doi:10.1016/j.media.2011.07.003.
12. Lee LC, Genet M, Acevedo-Bolton G, Ordovas K, Guccione JM, Kuhl E. A computational model that predicts reverse growth in response to mechanical unloading. Biomech Model Mechanobiol. 2015;14(2):217-229. doi:10.1007/s10237-014-0598-0.
Figures