4780
Automatic labeling of cerebral vessels in time-resolved contrast enhanced angiography1Functional Brain Center, The Wohl Institute for Advanced Imaging, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel, 2Sagol School of Neuroscience, Tel Aviv University, Tel Aviv, Israel, 3Department of Radiology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel, 4Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
Synopsis
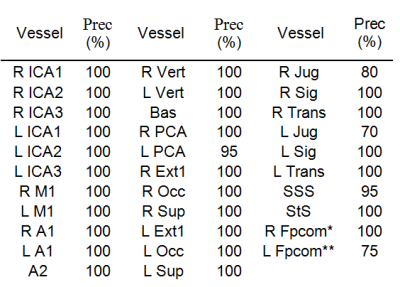
This study proposes a fully automatic labeling algorithm detecting up to 32 cerebral vessels including arteries and veins, based on 20 data sets of time-resolved contrast-enhanced angiography. The cerebral vessels include: internal carotid arteries (3-segments), middle cerebral arteries (M1), anterior cerebral artery (ACA,A1), ACA (A2), vertebral arteries, basilar artery, posterior cerebral arteries, external carotid, occipital arteries, superficial temporal arteries, internal jugular veins, and the sigmoid, transverse, superior sagittal and straight sinuses. The algorithm’s performance was validated on another 20 data sets demonstrating high precision including for the identification of the fetal posterior communicating artery, a common variant of the blood vessels.
PURPOSE
The ability to examine the vascular structure and flow dynamics is of central importance in the diagnosis and evaluation of many cerebrovascular diseases. Time-resolved contrast enhanced angiography presents a relatively large amount of data both on the spatial and temporal dimensions. A basic step in analyzing the vascular system is identifying the different blood vessels in the image, to allow for further processing. A few studies have been proposed for automatic labeling of the blood vessels in time-of-flight (TOF) and phase contrast (PC) methods [1-5]. However, these studies related to only 20 blood vessels at most, and TOF and PC have lower contrast-to-noise ratio, and require longer scan time than time resolved contrast enhanced angiography. The aim of this study was to develop a fully automatic algorithm to label the major blood vessels and their segments, both arteries and veins for Time-resolved Angiography With Interleaved Stochastic Trajectories (TWIST).METHODS
Subjects: Data of 40 subjects (ages 7-74) were included in this study. 20 data sets were used to develop the algorithm, and an additional 20 datasets were used to estimate the algorithm’s precision.
MR protocol: MR Scans were performed on 3T Prisma or Skyra Siemens scanners. TWIST data was acquired with coronal orientation, spatial resolution of 0.9-1X0.9-1X1mm3 and temporal resolution of 1.8-2.7sec. A power injector was used to infuse 0.2cc/kg (0.5M) of contrast agent (Gadolinum-Dotarem), followed by a flush of 20cc saline, at a constant rate of 2cc/sec. 25-54 measurements were acquired with a total scan time of 0:56-2:14 minutes.
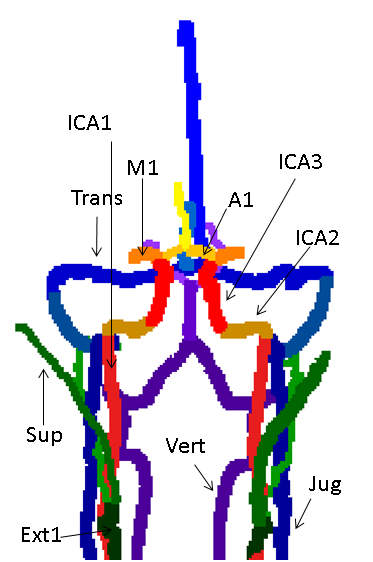
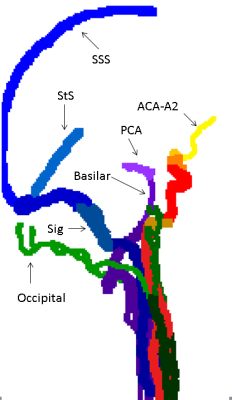
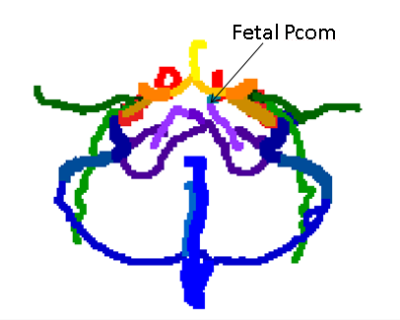
Labeling algorithm: The algorithm aims to automatically detect up to 32 vessel segments. The segments defined were: The right and left (R/L) internal carotid artery (ICA), each divided into 3 segments (extra-cranial, petrous and cavernous-supraclinoid), R/L M1 segment of the middle cerebral artery (MCA), R/L A1 segment of anterior cerebral artery (ACA), combined A2 segments of the ACA, R/L vertebral arteries, the basilar artery, R/L posterior cerebral artery (PCA), R/L external carotid arteries up to occipital bifurcation, R/L occipital arteries, R/L superficial temporal arteries, R/L internal jugular veins, R/L sigmoid sinuses, R/L transverse sinuses, superior sagittal sinus, straight sinus, and in case of fetal variant, R/L fetal posterior communicating arteries (Pcom).
The developed algorithm was based on specific properties of each blood vessel: their spatial location, bolus arrival time, segmentation threshold (dominance), shape, orientation and bifurcation points. First the major blood vessels were identified and the smaller vessels were detected gradually, using the previously detected vessels as reference points. Generally, for each vessel, the algorithm was based on the following steps: 1. Identification of the relevant time frame (e.g for the carotids – the time the bolus reaches the neck) 2. Creation of an image with values based on the vessel’s relevant time frames and removal of previously detected vessels and areas which are outside the estimated vessel area from the image. 3. Search for the maximum threshold which allows segmentation of the vessel – using region growing from a starting point until a target area is reached or a criterion on the orientation of the growing vessel is met. 4. Creation of a skeleton vessel; this was done using a skeletonization algorithm, which allows analysis of the segmented vessels as a graph [6,7]. Using the shortest path from starting voxel to target voxels allows the isolation of specific vessels and bifurcations of interest.
The precision of the algorithm was evaluated on 20 data sets using the formula: precision= (number of true identifications)/(number of true identifications + number of false identifications).
RESULTS & DISCUSSION
The algorithm was successfully applied to all data sets, with a mean run time of three minutes. Figures 1-3 show coronal, axial and sagittal projections of the detected vessels in different subjects. The precision of the algorithm was 100% in 27 out of 32 segments. Table 1 shows the algorithm’s precision in each segment (n=20). In a number of subjects the jugular veins were not fully apparent, resulting in lower precision for this vessel. The algorithm detected 7 cases of fetal Pcom, which were in agreement with TOF data, expect for one falsely identified Pcom.CONCLUSION
This study proposes a fully automatic labeling algorithm for time resolved contrast enhanced angiography. This sequence is a routinely used clinical scan taking about one minute of acquisition time during contrast agent injection. The algorithm automatically identifies up to 32 vessel segments, including major arteries and veins, and is an important basic step in order to analyze the structure and dynamics of the vascular system, information which is vital to enhance our understanding of cerebrovascular diseases.Acknowledgements
No acknowledgement found.References
[1] Bilgel, Murat, et al. "Automated anatomical labeling of the cerebral arteries using belief propagation." Proceedings of SPIE. Vol. 866918. NIH Public Access, 2013.
[2] Dunås, Tora, et al. "Automatic labeling of cerebral arteries in magnetic resonance angiography." Magnetic Resonance Materials in Physics, Biology and Medicine 29.1 (2016): 39-47.
[3] Robben, David, et al. "Simultaneous segmentation and anatomical labeling of the cerebral vasculature." Medical image analysis 32 (2016): 201-215.
[4] Bogunović, Hrvoje, et al. "Anatomical labeling of the circle of willis using maximum a posteriori probability estimation." IEEE transactions on medical imaging 32.9 (2013): 1587-1599.
[5] Wang, Xingce, et al. "Automatic labeling of vascular structures with topological constraints via HMM." International Conference on Medical Image Computing and Computer-Assisted Intervention. Springer, Cham, 2017
[6]Lee, Ta-Chih, Rangasami L. Kashyap, and Chong-Nam Chu. "Building skeleton models via 3-D medial surface axis thinning algorithms." CVGIP: Graphical Models and Image Processing 56.6 (1994): 462-478.
[7] Kerschnitzki, Michael, et al. "Architecture of the osteocyte network correlates with bone material quality." Journal of bone and mineral research 28.8 (2013): 1837-1845.
Figures