4770
Fast multi-slice myocardial T1 mapping (FAST1)1School of Biomedical Engineering and Imaging Sciences, Faculty of Life Sciences and Medicine, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
Myocardial T1 mapping shows promise for the detection of cardiomyopathy. The widely-used inversion recovery based approaches, such as MOdified Look-Locker Inversion recovery (MOLLI), can only generate a single T1 map from one breathhold scan. In this study, we developed a novel FASt multi-slice myocardial T1 mapping (FAST1) approach in a single breathhold using slice-selective inversion recovery, two inversion times per slice and an advanced reconstruction approach. This technique was evaluated in a phantom as well as in healthy volunteers, and provided similar repeatability, limited precision penalty and increased spatial coverage compared to MOLLI.
Introduction
Myocardial T1 mapping shows promise for detecting cardiomyopathy1-3. Inversion recovery based approaches such as MOdified Look-Locker Inversion recovery (MOLLI)1 are the most widely-used myocardial T1 mapping methods due to their high precision and repeatability4. In these approaches, a single T1 map is generated from one breathhold scan consisting of several non-selective inversion pulses, each followed by a series of ECG-triggered single-shot 2D acquisitions. In this study, we developed and characterized a novel FASt multi-slice myocardial T1 mapping (FAST1) approach using slice-selective inversion recovery, two inversion times per slice, and an advanced reconstruction approach.Methods
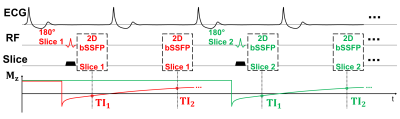
(1) Pulse Sequence: Fig. 1 illustrates the FAST1 pulse sequence diagram. Two ECG-triggered images of the same slice are acquired following a slice-selective inversion pulse. The timing between the slice-selective inversion pulse and imaging is minimized to reduce the impact of cardiac motion. Such imaging block is repeated for different slice locations within a breathhold acquisition. To improve robustness of the inversion against potential motion, the ratio of inversion to imaging slice thickness was set to 2.5. Slice-interleaved acquisition combined with the slice gap twice the imaging slice thickness was employed to minimize potential slice cross-talk.
(2) T1 Map Reconstruction:
T1 maps were reconstructed offline. T1 fitting was performed by exhaustive
search over a dictionary of a one-parameter model $$$(1-(1+α)e^{-TI/T1})$$$,
where α represents the inversion efficiency of the inversion pulse. By applying
Bloch equation simulations of the employed inversion pulse (phase-modulated
hyperbolic secant pulse) using typical myocardial T1/T2 times (T1=[400-1600]ms,
T2=50ms), α was calculated as 0.9353. During the fitting process, the measured
data $$$S_{meas}$$$ were scaled to each dictionary entry as: $$$S_{measScaled} = S_{meas} \cdot \overline{|S_{dict}|}
/ \overline{|S_{meas}|}$$$, where $$$\overline{|S_{dict}|}$$$ and $$$\overline{|S_{meas}|}$$$ are
the average signals over all inversion times of a dictionary entry and of the measured
signal, respectively. The polarity of the measured signal was restored using a multi-fitting approach.
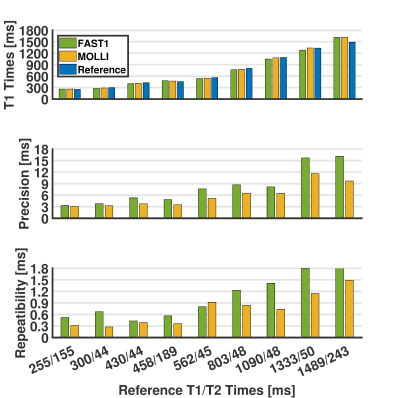
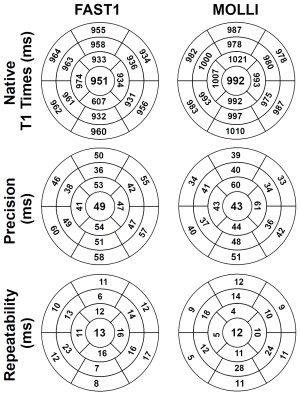
(3) Experiments and Data Analysis: All experiments were performed on a 1.5T MR scanner (MAGNETOM Aera, Siemens Healthcare). FAST1 was compared to MOLLI (5-(3)-3 scheme) in phantom (T1MES, Resonance Health) with nine vials of different T1/T2 times5 and in four healthy volunteers for native T1 mapping. Both sequences used the same single-shot 2D bSSFP imaging parameters: TR/TE/FA 2.7ms/1.1ms/35°, FOV 360×306mm2, voxel size 1.4×2.1mm2, slice thickness 8mm, GRAPPA factor 2, partial Fourier factor 7/8, bandwidth 1085Hz/px, shortest inversion time 100ms. Fifteen slices were acquired using FAST1 in three 10-heartbeat breathholds (each with 5 slices) to provide whole heart coverage and three slices using MOLLI in three separate 11-heartbeat breathholds. Each approach was repeated five times for the phantom, and twice for each volunteer. Measurements, precision, and repeatability of T1 estimates were calculated vial-wise for the phantom, as well as segment-wise (using a 16-myocardial-segment AHA model6).
Results
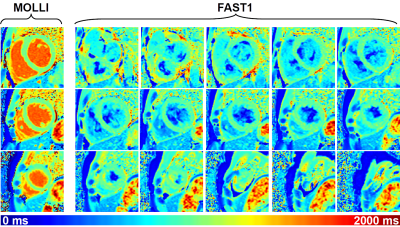
For the phantom, FAST1 provided reduced T1 times (p=0.04), reduced precision (8.2±4.8ms vs. 5.9±3.0ms, p<0.01), and reduced repeatability (1.0±0.5ms vs. 0.7±0.4ms, p=0.01) compared to MOLLI (cf. Fig. 2). For the volunteers, FAST1 provided lower in-vivo native myocardial T1 times (951±15ms vs. 992±13ms, p<0.01), slightly reduced precision (49±7ms vs. 43±8ms, p=0.02) and similar repeatability (13±4ms vs. 12±6ms, p=0.69) compared to MOLLI (cf. Fig. 3). Example native T1 maps using both methods on a healthy volunteer are shown in Fig. 4, where comparable native myocardial T1 map quality with a similar range of T1 times was achieved using FAST1 (fifteen slices covering the whole heart, acquired in three 10-heartbeat breathholds) and MOLLI (three slices in three 11-heartbeat breathholds).Discussion
Compared to standard MOLLI sequences, FAST1 provides improved spatial coverage of the heart by enabling multi-slice native myocardial T1 mapping, similar in-vivo repeatability and limited in-vivo precision penalty of 15%. The three separate breathholds in standard clinical protocols of myocardial T1 mapping using three-slice coverage could then be reduced to one breathhold using FAST1. Alternatively, FAST1 can offer whole-heart T1 mapping in three breathholds. FAST1 is not suitable for quantification of blood T1 times due to the in-flow effect caused by the slice-selective inversion pulse. Although extracellular volume mapping is thus currently not feasible using this technique, the acquisition of an additional single-slice T1 map with a non-selective inversion pulse will be investigated in the future for blood T1 and extracellular volume quantification.Conclusion
FAST1 enables multi-slice myocardial T1 mapping in a single breathhold with limited precision penalty, similar repeatability, and increased slice coverage of the heart compared to MOLLI.Acknowledgements
This work was supported by the Health Innovation Challenge Fund [Grant number HICF-R10-698], a parallel funding partnership between the Department of Health and the Wellcome Trust, the Wellcome EPSRC Centre for Medical Engineering at King's College London (WT 203148/Z/16/Z), and the EPSRC grant (EP/R010935/1). This research was also supported by the National Institute for Health Research (NIHR) Biomedical Research Centre award to Guy's and St Thomas' NHS Foundation Trust in partnership with King's College London, and by the NIHR Healthcare Technology Co-operative for Cardiovascular Disease at Guy’s and St Thomas' NHS Foundation Trust.References
[1] Messroghli DR, et al. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52(1):141-146.
[2] Messroghli DR, et al. T1 mapping in patients with acute myocardial infarction. J Cardiovasc Magn Reson. 2003;5(2):353-359.
[3] Moon JC, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15(1):92.
[4] Roujol S, et al. Accuracy, precision, and reproducibility of four T1 mapping sequences: a head-to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology. 2014;272(3):683-689.
[5] Captur G, et al. A medical device-grade T1 and ECV phantom for global T1 mapping quality assurance - the T1 Mapping and ECV Standardization in cardiovascular magnetic resonance (T1MES) program. J Cardiovasc Magn Reson. 2016;18(1):58.
[6] Cerqueira MD, et al.
Standardized myocardial segmentation and nomenclature for tomographic imaging
of the heart. A statement for healthcare professionals from the Cardiac Imaging
Committee of the Council on Clinical Cardiology of the American Heart
Association. Circulation. 2002;105(4):539-542.
Figures