4769
Quantitative modeling and detection of flux through pyruvate dehydrogenase in human myocardium1Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 2Simmons Cancer Center, UT Southwestern Medical Center, Dallas, TX, United States, 3Internal Medicine, UT Southwestern Medical Center, Dallas, TX, United States, 4University of Toronto, Toronto, ON, Canada
Synopsis
Flux through pyruvate dehydrogenase, a key regulatory enzyme, may be detected in human myocardium by hyperpolarization (HP) technology. A previously-validated model for quantitation of pyruvate metabolism in isolated hearts was extended to human myocardium. HP [13C]bicarbonate and HP[1-13C]lactate were detected from heart muscle after injection of ~0.43 mL/kg of 250mM HP[1-13C]pyruvate in four healthy subjects. All subjects tolerated the procedure well. Kinetic modeling yielded a rate constant for oxidation of pyruvate to bicarbonate of 0.005 sec-1, lower than that reported for isolated rodent hearts. Assessment of PDH flux in human myocardium is feasible with hyperpolarization technology.
Introduction
Pyruvate dehydrogenase (PDH) is a large multienzyme complex that regulates cardiac metabolism by balancing carbohydrate and fatty acid oxidation. Noninvasive detection of flux through PDH in the human heart would unquestionably provide valuable information about heart disease. Pharmacological interventions that increase PDH activity have been examined for > 50 years with the goal of protecting ischemic myocardium and improving function in the failing heart, yet their clinical role is controversial, in part because methods to specifically detect PDH are not applicable in human patients. The recent availability of hyperpolarization (HP) technology enables monitoring of PDH activity in isolated hearts (1, 2), rodents in vivo (3) and in human subjects (4). Computational models to determine fluxes through key pathways in pyruvate metabolism have been proposed and validated in isolated hearts (5). Clinical development of this technology will benefit from extracting quantitative metabolic information from the dynamic 13C MRS data. We tested the hypothesis that a quantitative metabolic model (5) for evaluation of HP[1-13C]pyruvate metabolism in perfused rat heart could be translated for use in humans.Methods
Four healthy adult human subjects (1 female, 3 male) were studied under an IRB-approved protocol. Subjects fasted overnight, ate a small breakfast, and were given 35 grams of glucose orally about 45 min prior to the exam. HP methods followed earlier reports (4, 6). Briefly, HP[1-13C]pyruvate was prepared in a SPINLab®. After quality control evaluation including testing of the integrity of the final sterilization filter and release by a pharmacist, ~0.43 mL/kg of 250 mM HP[1-13C]pyruvate was injected intravenously, followed by a saline flush. Spectral data acquisition (2 subjects) or spectral-spatial imaging, followed by acquisition of spectral data (2 subjects) was performed with ECG gating. The spectral-spatial imaging was described previously (4). A “clamshell“ transmit and 8 channel “paddle” receive arrays were used (GE Healthcare). Only data from the 4 channels anterior to the heart were used in analysis of the kinetic data presented here. Dynamic metabolism was modeled using the system of non-linear differential equations described in (5). Numerical solutions to the system was written in MATLAB to determine first order rate constants that characterize the metabolic conversion of pyruvate to lactate (kPL) and pyruvate to bicarbonate (kPB). T1s were assumed based on earlier reports (5). The dynamic signal intensities of HP[1-13C]pyruvate, HP[1-13C]lactate and HP[13C]bicarbonate were obtained using singular value decomposition.Results and Discussion
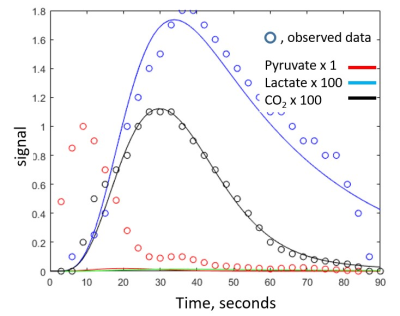
The four subjects tolerated the exam without incident, and physical findings and laboratory work were unchanged. An example of a short axis [13C]bicarbonate image is shown in Figure 1 and a typical complete dynamic data set is shown in Figure 2. Dynamic data including HP[1-13C]pyruvate, HP[1-13C]bicarbonate and HP[1-13C]lactate could not be fit by the model, in contrast to results from isolated hearts. However, elimination of the HP[1-13C]pyruvate data enabled acceptable fitting of the HP[1-13C]lactate and HP[13C]bicarbonate data. Based on the imaging data the great majority of HP[1-13C]pyruvate signal in nonselective spectral acquisition after venous injection results from pyruvate in the right and left ventricular cavity. In isolated hearts, the injection is arterial. Figure 3 illustrates typical fitting of the HP[13C]bicarbonate and HP[1-13C]lactate signals. The data consistently demonstrated production of small (compared to HP[1-13C]pyruvate) but analyzable dynamic HP[1-13C]lactate and HP[13C]bicarbonate signals that result from active myocardial metabolism. The calculated rate constant for conversion of [1-13C]pyruvate to [13C]bicarbonate was 0.005 ± 0.004 sec-1, rates that are approximately an order of magnitude slower than typically measured in perfused rat heart and consistent with known differences between in vivo human heart and isolated perfused rat heart. These results should be interpreted with caution for a number of reasons, as follows: 1) T1 decays were assumed and may be difficult to measure in a multicompartment system, for example T1 of intramitochondrial 13CO2; 2) the derivative of the input function of [1-13C]pyruvate was assumed to be gamma variate; 3) the contribution of HP[1-13C]lactate was assumed to arise primarily from the myocardium but blood contribution may be significant; 4) the mathematical model assumes a single compartment of metabolism, and 5) actual flux measurements rely on [pyruvate] in mitochondria.Conclusion
Standard radionuclide imaging methods do not provide information about individual reactions because the measured signal represents the weighted sum of the tracer plus the biochemical products produced by tissue. 13C NMR spectroscopy is far more powerful because fluxes in a single enzyme complex can be detected in human myocardium.Acknowledgements
EB015908References
1. Merritt ME, Harrison C, Storey C, Jeffrey FM, Sherry AD, Malloy CR. Hyperpolarized 13C allows a direct measure of flux through a single enzyme-catalyzed step by NMR. Proc Natl Acad Sci U S A. 2007; 104; 19773-19777. PMID: 18056642
2. Schroeder MA, Atherton HJ, Ball DR, Cole MA, Heather LC, Griffin JL, Clarke K, Radda GK, Tyler DJ. Real-time assessment of Krebs cycle metabolism using hyperpolarized 13C magnetic resonance spectroscopy. FASEB J. 2009; 23: 2529-38. PMID: 19329759
3. Schroeder MA, Cochlin LE, Heather LC, Clarke K, Radda GK, Tyler DJ. In vivo assessment of pyruvate dehydrogenase flux in the heart using hyperpolarized carbon-13 magnetic resonance. Proc Natl Acad Sci U S A. 2008; 105: 12051-6. PMID: 18689683
4. Cunningham CH, Lau JY, Chen AP, Geraghty BJ, Perks WJ, Roifman I, Wright GA, Connelly KA. Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circ Res. 2016; 119: 1177-1182. PMID: 27635086; PMCID: PMC5102279.
5. Mariotti E, Orton MR, Eerbeek O, Ashruf JF, Zuurbier CJ, Southworth R, Eykyn TR. Modeling non-linear kinetics of hyperpolarized [1-(13) C] pyruvate in the crystalloid-perfused rat heart. NMR Biomed. 2016; 29: 377-86. PMID: 26777799; PMCID: PMC4832359.
6. Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, Ardenkjaer-Larsen JH, Chen AP, Hurd RE, Odegardstuen LI, Robb FJ, Tropp J, Murray JA. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Sci Transl Med. 2013; 5: 198ra108. PMID: 23946197
Figures
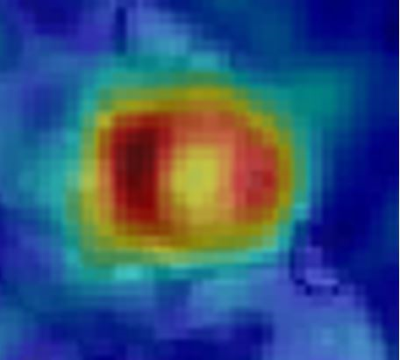
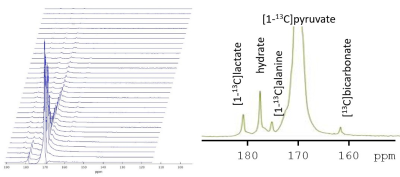
Figure 2. Stacked plot of kinetic data. Each spectrum is from a single anterior channel with a temporal resolution of ~3 seconds. Localization was determined by the receive profile of the coil. A single spectrum is shown in the inset.