4764
Tracking of PLGA-PFCE-labeled Cardiac Stem Cells Seeded on Novel Biodegradable Poly(3-hydroxyoctanoate) Scaffolds Implanted on the Murine Myocardium using 1H and 19F MRI/MRS1U. Oxford, Oxford, United Kingdom, 2U. Westminster, London, United Kingdom, 3Radboud University Medical Center, Nijmegen, Netherlands
Synopsis
Controlled administration of cardiac progenitor stem cells (CPCs), and their visualization and tracking and release, still present tremendous challenges in cellular/tissue therapy, yet fundamental and necessary tasks towards a therapeutically successful approach. In this work, we propose the synthesis and use of novel, functional biodegradable, biocompatible, Polyhydroxyalkanoate (PHA) and Poly-caprolactone (PCL) polymer blend scaffolds to: a) achieve controlled delivery of CPCs, thereby prolonging the viability, and maximizing retention of delivered stem cells to the murine myocardium, and b) the use of 19F MRI/MRS to noninvasively detect, and monitor the cells temporally.
Introduction
Stem cell delivery has been applied as a novel and promising therapeutic approach for cardiac disease [1–3]. Nevertheless, controlled administration of cardiac progenitor stem cells (CPCs) and their visualization and tracking still present tremendous challenges towards a therapeutically successful cell engineering approach. We propose the synthesis and use of novel, functional biodegradable, biocompatible, medium chain length Polyhydroxyalkanoate (MCL-PHA) and Poly-caprolactone (PCL) polymer blend scaffolds to: a) achieve controlled delivery of CPCs to the murine myocardium to maximize retention, and b) noninvasively detect and monitor the cells temporally using 19F MRI/MRS.
Methods
Production of MCL-PHA: MCL-PHA was produced by P. mendocina [4] using batch fermentation in a bioreactor and extracted in accordance to standard methodologies [4]. Porosity was induced using NaCl particles (75, 100 μm). Cell Isolation-Labeling: CPCs were isolated from adult, C57BL/6, GFP positive and negative, mouse atria in accordance to standard methods [5, 6]. Cells were then incubated with Perfluoro-crown-ether (PFCE)-containing fluorescent nanoparticles (NPs) (containing Atto647) (10 mg/mL in 1 million cells) and FuGENE (Promega, Madison, WI, USA) for approximately 24 h before trypsinization, isolation, and pelleting. Final cell pellet suspensions (106 cells) were transferred to 1.7 ml Eppendorf tubes in IMDM media. Cell Seeding: Labeled cells were seeded on scaffolds overnight. The respective cell densities were 500k/350k for in vitro/in vivo MRI studies (scaffold sizes were 2-8×2-8 mm2). To maximize cell density, a double-layered scaffold was implanted on a second mouse. MRI/MRS: All experiments were conducted on a 9.4 T Agilent (Agilent Technologies, USA). Radiofrequency (RF) Coils: An eight-rung quadrature birdcage (diameter=34 mm), and a 40×20 mm2 butterfly were constructed, tuned, and matched at 375.8 MHz for 19F-MRI. The broad frequency response of the coil allowed intermittent imaging on the 1H and 19F nuclei. In vitro MRI: 1H MRI was conducted with conventional SPGR and steady steady free precession (SSFP) sequences with repetition time (TR)/echo time (TE)=20-65.1/4 ms/flip angle=20°/number of excitations (NEX)=1-6/field-of-view (FOV)=40×40 mm2/slice thickness (ST)=2 mm (single/multislice acquisitions)/bandwidth (BW)=50 kHz/total acquisition time=7-50 s. Correspondingly, 19F MRI acquisitions (SPGR, SSFP) used TR/TE=8.3/4.2 ms/flip angle=50°/NEX=1024/matrix=32×32/ST=10 and 40 mm/BW=4 kHz/total acquisition time=4.3 min. Post-mortem MRI studies: The 2D 1H MRI acquisition parameters were: TR/TE=2.73 or 3.13/1.6 ms/flip angle=50°/NEX=32/matrix=128×128/ FOV=40×40 mm2/ST=1 mm/BW=100 kHz, total acquisition time = 12.8 s. The 3D 1H MRI acquisition parameters were: TR=2.63 ms or 2.73 ms/TE=1.33 or 1.38 ms/flip angle=20°/NEX=4/matrix=128×128×128/FOV=40×40×40 mm3/BW=100 kHz/total acquisition time=2.5 min, while 19F parameters were: TR/TE=8.3/4.2 ms/flip angle=50°/NEX=796/matrix=32×32/ST=5 mm/BW=4 kHz, total acquisition time=3.31 min. Nonlocalized 19F MRS was acquired using TR=800 ms/NEX=256/512 points/BW=20 kHz/RG=30. In vivo murine studies: 3D ungated scans were acquired from two anesthetized mice (under 1.5-2% isoflurane) using TR/TE=3/1.7 ms/flip angle=30°/NEX=4/matrix=128×128×128/FOV=40×40×40 mm2/BW=100 kHz/total acquisition time~5 min. 19F MRI/MRS: 19F spectra were acquired using nonlocalized acquisitions (ungated and gated for in vivo scans) with TR=800-1000 ms/NEX=64 or 256, 512 points, and BW=20 kHz. Image Processing: Images were imported and interpolated in ImageJ (NIH, Bethesda, USA) using bicubic spline interpolation to match the 1H matrix size, and both 1H/19F MRI were overlaid (opacity=30-70%).Results
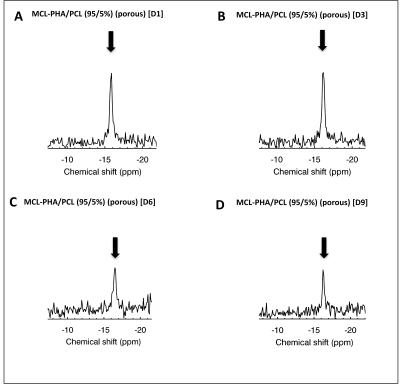
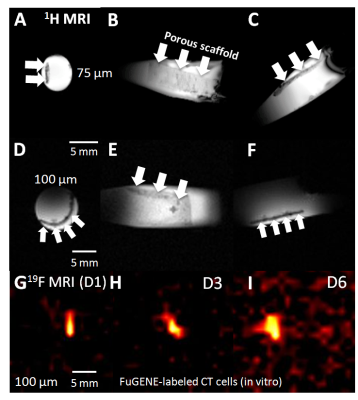
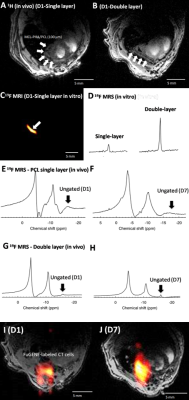
Figs. 1 and 2 show 19F MRS/MRI over a temporal period of 9 days (D1-D9) following seeding of FUGENE-labeled cells. Constancy of cell retention is justified by the 25% integrated MRS area difference (D1 vs. D3). The cell density decreased to 44% at D6 and 30% at D9. Decreases are likely attributed to cell death and/or decreased cell adherence that led to increased leaching rates of non-viable/viable cells from the scaffold. MRI also achieved in vitro scaffold visualization using 1H/19F MRI (Fig. 2). Indicative is the decreased signal response (D6 vs. earlier days) (Fig. 2 G-I). The seeded scaffolds were successfully tested in the post-mortem mouse using both 19F MRS/MRI (not shown). In vivo tests conducted in two C57BL/6 mice indicate the ability of 19F MRS to detect and track the labeled, seeded cells within a week (Fig. 3A-B, E-J). The scaffold was also distinctly identified in 1H MRI at D1 and D7 in the case of the double-layered scaffold (the detected 19F spectral area doubled vs. the single-layered scaffold (Fig. 3I, J)). The single-layered scaffold was not visible using 19F MRI as a result of the low seeding density (~300k at D1).Discussion
We have presented applicability of MCL-PHA/PCL porous blends fabricated as thin films with an improved performance compared to MCL-PHAs, capitalizing on the excellent polymeric properties of natural MCL-PHA polymers, and the simplified synthetic process relevant to PCLs. We have also demonstrated in vivo applicability and MRI visualization over periods spanning 8 days in the in vivo murine heart.Acknowledgements
We are thankful to Ms. A. Vernet for her help with the in vivo studies and Dr. A. Shaw for his help with the histological imaging. We thank Dr. M. Maguire for the permission to use the solenoid coil for some of the in vitro studies. We thank Professor Jonathan Knowles for providing access to the contact angle measurement and SEM facilities at the Eastman Dental Institute.
The work was supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie [grant agreement No. 652986], and the European Research Council Grant ERC-2013-StG-336454 (MS).
References
1. S.A.J Chamuleau, K.R. Vrijsen, D. G. Rokosh, X.L. Tang, J.J. Pick, R. Bolli, Cell therapy for ischaemic heart disease: Focus on the role of resident cardiac stem cells, Neth Heart J 17(2009) 199-207.
2. A.P. Beltrami, L. Barlucchi, D. Torella, M. Baker, L. Federica, S. Chimenti, H. Kasahara, M. Rota, E. Musso, K. Urbanek, A. Leri, J. Kajstura, B. Nadal-Ginard, P. Anversa, iAdult cardiac stem cells are multipotent and support myocardial regeneration, Cell 114 (2003) 763-776.
3. Oh H, S.B. Bradfute, T.D. Gallardo, T. Nakamura, V. Gaussin, Y. Mishina, J. Pocius, L.H. Michael, R.R. Behringer, Garry DJ, M.L. Entman, M.D. Schneider, Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction, PNAS 100 (2003) 12313-8.
4. R. Rai, T. Keshavarz, J.A. Roether, A.R. Boccaccini, I. Roy, Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future, Materials Science and Engineering 72 (2011) 29-47.
5. S. Malandraki-Miller, R. Tyser, P. Riley P, C.A. Carr, Comparative characterization of cardiac atrial progenitor cell populations for use in cell therapy, Heart 100 (2014) A14.
6. S.C. Tan, R.S. Gomes, K.K. Yeoh, F. Perbellini, S. Malandraki-Miller, L. Ambrose, L.C. Heather, G. Faggian, C.J. Schofield, K.E. Davies, K. Clarke, C.A. Carr, Preconditioning of cardiosphere-derived cells with hypoxia or prolyl-4-hydroxylase inhibitors increases stemness and decreases reliance on oxidative metabolism, Cell Transplant 25(1) (2016) 35–53 doi: 10.3727/096368915X687697.
Figures