4763
5D cardiac MRI on mouse models : preliminary results1CRMSB UMR5536, CNRS-Univ.Bordeaux, Bordeaux, France
Synopsis
Until now, the data acquired during breathing period are not used to obtain 4D cardiac images on mouse models. Consequently, the purpose of the work presented here is to evaluate the impact of breathing on the movement of the mouse's heart and to develop a method for reconstructing the cardiac self-gating signal during respiration. Finally, preliminary results of 5D image reconstruction (3D images during the heart rate and according to respiratory movement) will be shown in mice.
INTRODUCTION
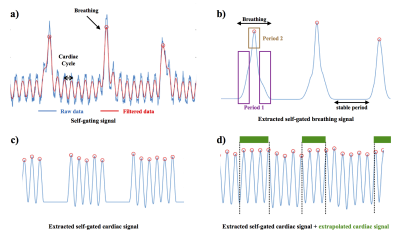
Instrumental developments and the use of non-Cartesian MRI sequences have recently resulted in the ability to obtain 4D cardiac images on mouse models1,2,3. Until now, the effect of breathing is often neglected or the data acquired during this period are not used. Indeed, unlike respiration in humans, the breathing of an anesthetized mouse takes the form of a start or a « hiccup ». As ECG or self-gating data are not usable during this period (Figure 1.a), The acquisition time of 4D cardiac images in small animals remains long and the signal-to-noise ratio limited. There is therefore an interest to use data acquired during breathing to improve 4D cardiac imaging on small animal models. For this purpose, we developed a strategy enabling to obtained 5D cardiac imaging in mice. This strategy relies on the use of a self-gated UTE 3D sequence with a pseudo-random encoding. The sequence was combined with the injection of iron nanoparticles to enhance blood signal. Therefore, we were able to evaluate the impact of breathing on the movement of the mouse's heart and to develop a method for reconstructing the cardiac self-gating signal during the breathing periods. Finally, preliminary results of 5D images (3D images as a function of the heart rate and according to the respiratory movement) in the animal.METHODS
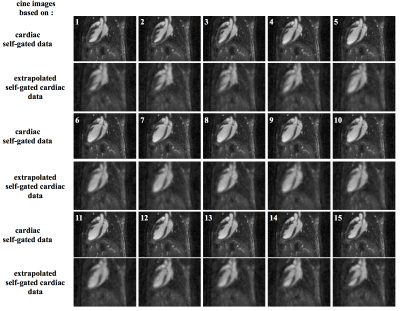
MRI experiments were performed on a 7T Bruker (Germany) equipped with a volume resonator for transmission, and a four-element phased array surface coil for signal reception. C57BL/6 mice (N=8) were anesthetized with isoflurane. Before mice positioning in the magnet, a 100 μL volume of 200 μmol Fe/kg of ultrasmall superparamagnetic iron oxide (USPIO) was injected through the tail vein. The ECG signal was picked up using electrodes wrapped around the forelimbs. Cardiac rhythm was stabilized around 450-475 beats/min. A 3D self-gated UTE sequence with the following parameters was used : TR/TE=3.5/0.305 ms, Flip angle=20°; Pulse duration=0.05ms; 3 data points sampled for navigator signal; 30 000 projections distributing according to the Golden angle method. Number of Repetitions=10 corresponding to a total acquisition time of 17min. A Gaussian filter was applied to the self-gating data of the 4 coils. From these data, the respiratory signal and the cardiac signal were extracted separately (Figure 1.b and c). The images according to the respiratory rhythm were reconstructed and the displacement of the heart was measured along the 3 axes of space. Cardiac data were extrapolated during peak respiration from standard data (Figure 1.d). Cine-cardiac images were reconstructed during breathing.RESULTS
The images according to the respiratory rate enabled to measure an average displacement of 8 pixels along the vertical axis and 6 pixels along the horizontal axis on the 8 mice. This displacement occured during the duration of the expiratory phase (approximately 28 ms). This period could be decomposed into 2 (period 1 and 2 in Figure 1.b) for future 5D image reconstruction. Heart movements were negligible during the stable period. The data obtained as a function of the heart rate and during the stable phase of respiration were used to reconstruct 3D cine-cardiac images. Fifteen images were reconstructed by cardiac cycle with a signal-to-noise ratio of 34±3. Cardiac data extrapolated during the breathing phase were also used to reconstruct cine-cardiac 3D images. The number of projections to reconstruct these data was approximately 1218±315 by cardiac images. The images obtained show a signal-to-noise ratio and a spatial resolution degraded compared with the other data (Figure 2).DISCUSSION
The work presented here shows that breathing has a significant impact on the position of the heart of the animal during the acquisition. The analysis of this position enabled to establish the existence of 3 phases in mice: a stable phase, a transition phase and an inhalation phase. Usually the cine-cardiac data is reconstructed only during the stable phase. The self-gating cardiac data during the stable phase can be extrapolated to obtain a cardiac self-gating signal during the breathing period. Cine-cardiac images can thus be reconstructed in 5 dimensions.CONCLUSION
Here, the self-gating data obtained with a 3D UTE sequence can be used to reconstruct images of the mouse heart, depending on respiration, heart rate or both. The method proposed here should improve the signal-to-noise ratio of cardiac images in animals or further reduce the acquisition time.Acknowledgements
This work was supported by a public grant, Translational Research and Advanced Imaging Laboratory, which is part of the French National Research Agency’s Investments for the Future Program (“NewFISP”; ANR- 10-LABX-57).References
1. Four-dimensional MR microscopy of the mouse heart using radial acquisition and liposomal gadolinium contrast agent. Bucholz E, Ghaghada K, Qi Y, Mukundan S, Johnson GA. Magn Reson Med. 2008 Jul;60(1):111-8. doi: 10.1002/mrm.21618.
2. USPIO-enhanced 3D-cine self-gated cardiac MRI based on a stack-of-stars golden angle short echo time sequence: Application on mice with acute myocardial infarction. Trotier AJ, Castets CR, Lefrançois W, Ribot EJ, Franconi JM, Thiaudière E, Miraux S. J Magn Reson Imaging. 2016 Aug;44(2):355-65. doi: 10.1002/jmri.25150.
3. Positive contrast high-resolution 3D-cine imaging of the cardiovascular system in small animals using a UTE sequence and iron nanoparticles at 4.7, 7 and 9.4 T. Trotier AJ, Lefrançois W, Van Renterghem K, Franconi JM, Thiaudière E, Miraux S. J Cardiovasc Magn Reson. 2015 Jul 7;17:53. doi: 10.1186/s12968-015-0167-4.
Figures