4760
Practical implementation of SMS bSSFP in the Heart1Biomedical Engineering, University of Southern California, Los Angeles, CA, United States, 2Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 3Department of Electrical Engineering, University of Southern California, Los Angeles, CA, United States
Synopsis
Cardiac MRI frequently relies on balanced stead-state free precession (bSSFP) for its beneficial contrast and SNR efficiency. Simultaneous multi-slice (SMS) imaging, specifically blipped controlled aliasing in parallel imaging, faces two major challenges with cardiac bSSFP: 1) artifacts from non-uniform signal in the through-slice direction and 2) spuriously excited side-lobes from imperfect multi-band excitation. We carefully evaluate both challenges using simulations and phantom experiments, and demonstrate practical SMS bSSFP imaging with 3-slice coverage (apical, mid, and basal short axis slices) at 3 Tesla, with good image quality in both transient-state and steady-state.
Purpose
Single slice balanced steady state free precession (bSSFP) imaging is widely used in cardiac MRI for myocardial function, perfusion, and relaxometry [1]. Complete assessment of the left ventricle is important. The American Heart Association (AHA) recommends a 17-segment model with 3 short axis slices and 1 long axis slice to evaluate biomarkers of myocardial disease [2]. This study investigates simultaneous multi-slice (SMS) cardiac bSSFP imaging. SMS imaging has been demonstrated for cardiac EPI [3] and GRE [4]. However these may not be ideal for quantitative imaging and bSSFP may be a better choice due to its superior SNR efficiency [5]. We specifically characterize two limitations that are specific to blipped-controlled aliasing in parallel imaging (CAIPI) SMS bSSFP in the heart:
1-- Artifacts caused by gradient z-encoding due to unresolved linear phase in areas with non-uniform signal in the through-slice direction (NUST) [3]; and
2-- Spurious side-lobe excitations caused by RF transmission imperfections [6,7] due to short, high flip-angle RF pulses used in cardiac bSSFP imaging.
Methods
Simulations were performed in MATLAB. Experiments were performed on a 3T GE Signa HDxT scanner using an 8-channel cardiac coil. Unless otherwise stated, fully-sampled bSSFP imaging was performed using TR/TE/FA: 3.2ms/1.5ms/30º, matrix size=96x96, and a 3-band RF pulse with duration/TBW: 0.65ms/2, center-to-center slice-spacing=2 cm, and slice-thickness=0.5 cm.
NUST Experiment
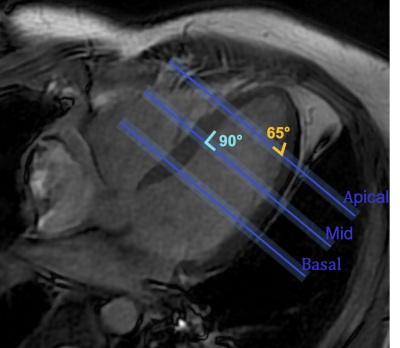
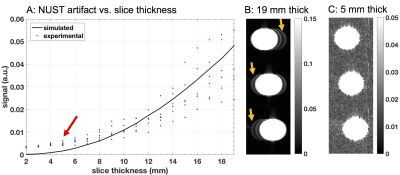
A uniform tube water-phantom was imaged to characterize the effect of slice thickness on the maximum NUST artifact in the myocardium. The phantom was tilted at an angle from the slice-select direction to match the maximum slice non-uniformity observed in the heart (Figure 1). Slice thickness was varied from 2-19mm and NUST artifact was calculated as the mean signal from the undesired slices. Values were normalized with respect to mean signal of the 19mm thick center slice. Simulations were performed to verify that NUST artifact was the result of non-uniform slice thickness. A digital phantom of same dimensions as the one used in the experiments was generated. Perfect excitation and linear phase were assumed.
Side-lobe Excitation Experiment
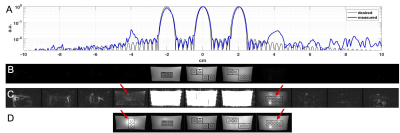
An 11 layer lego phantom (unique layers, in water) was imaged using 11 encoded slices. Z-profile was calculated from the middle PE of a bSSFP imaging sequence with the readout gradient moved to the slice select direction. Reconstructed images were compared to the z-profile of the RF pulse to visualize signal contribution from desired and spuriously-excited slices.
In-vivo experiments
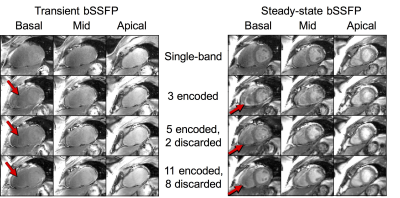
In-vivo experiments were performed on a healthy volunteer (M, 28) with written informed consent and Institutional Review Board approval. Apical, mid, and basal short axis slices were acquired with 3, 5, and 11 encoded slices to visualize artifact locations and potential for resolving artifact with extra encoded slices. Reconstructed images were compared to single-band reference slices.
Results
Figure 2 illustrates the close match between simulated and experimentally measured NUST artifact in the tube phantom. NUST artifact is indistinguishable from background noise for slice thicknesses <0.5 cm and becomes more severe with increased slice thickness.
Figure 3 illustrates the side-lobe performance in the lego phantom. Max side-lobe was <3.8% of the main-lobe. Side-lobe signal was highest at z=4cm and the unique layer at this location was able to be imaged.
Figure 4 shows 11 encoded slices and reference slices in a healthy volunteer. The multiband and single-band reference images were of comparable quality, with the exception of some residual artifact that can be identified when encoding additional slices.
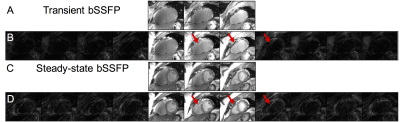
Figure 5 shows transient and fully sampled 3-band
bSSFP imaging with 3, 5, and 11 encoded slices. Similar to the lego phantom,
multiband and single-band images were of comparable quality, with the exception
of some residual artifact. Image quality improved with more encoded slices in both
transient and steady-state.
Conclusions
SMS cardiac bSSFP imaging is challenging due to the cardiac anatomy necessitating thick slices with relatively small gap, and the need for very high flip angles with short RF duration. Two acquisition constraints are characterized. We successfully demonstrate multiband cardiac images in transient and steady-state, adequate to simultaneously cover 16 of 17 LV myocardial segments, with good image quality. Our next step is to test undersampled SMS cardiac bSSFP using established evaluation metrics such as g-factor and slice-leakage [8].Acknowledgements
National Institutes of Health #R01-HL130494References
[1] Pennell D, Cardiovascular Magnetic Resonance, Circulation, Vol 121; p. 692-705, 2010
[2] Cerqueira M et. al., Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart: a statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American Heart Association, Circulation, Vol 105; p. 539-542, 2002
[3] Setsompop K et. al., Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med, Vol 67; p. 1210-1224, 2012
[4] Breuer F et. al., Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging, Magn Reson Med, Vol 53; p. 684-691, 2005
[5] Kober F et. al., Myocardial arterial spin labeling. J Cardiovasc Magn Reson, p. 18-22, 2016
[6] Zhu K et. al., RF Amplifier Nonlinearity Correction for Multiband RF pulses, 23rd ISMRM; p. 3763, 2015
[7] Chan F et. al., Effects of RF amplifier distortion on selective excitation and their correction by prewarping, Magn Res Med, Vol 23; p. 224-238, 1992
[8] Cauley S et. al., Interslice leakage artifact reduction technique for simultaneous multislice acquisitions, Magn Reson Med, Vol 72; p. 93-102, 2014
Figures