4759
Proton magnetic resonance spectroscopy to assess lipid content in cardiac amyloidosis1Institue for Biomedical Engineering, ETH Zürich, Zürich, Switzerland, 2Department of Cardiology, University Heart Center, University Hospital Zurich, Zürich, Switzerland, 3Department for Cardiology, Pneumology and Angiology, Heinrich Heine University, Düsseldorf, Düsseldorf, Switzerland, 4Institute of Diagnostic and Interventional Radiology, University Hospital Zürich, Zürich, Switzerland
Synopsis
Amyloidosis is a multisystemic disorder frequently affecting the heart and causing heart failure. In this work, MR imaging and spectroscopy was implemented and applied to characterize myocardial structure and function as well as changes in fatty acid storage of the heart. We found that myocardial triglyceride-to-water ratio was significantly decreased in amyloidosis compared to age-and body mass index-matched controls. Myocardial triglyceride-to-water ratio showed a negative tendency with increasing markers of heart failure. It is concluded that proton spectroscopy may provide an additional biomarker to gauge progression of cardiac amyloidosis.
Introduction
Amyloidosis is a multisystemic disorder that is characterized by extracellular deposition of misfolded amyloid proteins causing consecutive organ failure (1). Cardiac involvement is seen with two major forms of inherited or acquired amyloidosis, light-chain (AL) and transthyretin-related (ATTR) disease, leading to deteriorated prognosis and outcome (2). Cardiovascular Magnetic Resonance (CMR) is increasingly used to investigate patients with suspected cardiac involvement of amyloidosis. Typical cardiac manifestations include myocardial thickening, diffuse late gadolinium enhancement and heart failure resulting from expanded extracellular space (1). Recently, CMR has been shown to characterize different forms of amyloidosis (3). However, potential metabolic alterations involved have not been studied in detail. Previous histological and proton spectroscopy studies tend to yield opposing results in terms of myocardial lipid content in general heart failure and amyloidosis, therefore warranting further characterization (4,5). The objective of the present study was to assess myocardial triglyceride (TG) content along with cardiac function parameters in a group of patients with cardiac amyloidosis relative to age-matched controls.Methods
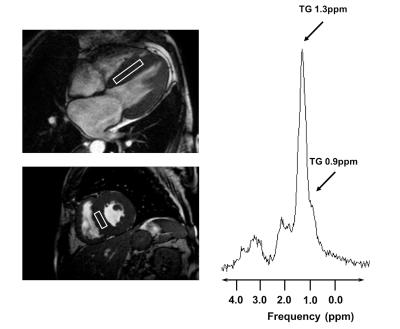
Imaging and spectroscopy was performed on a 1.5T system (Achieva, Philips, Best, Netherlands) using a 5-channel coil in patients with cardiac amyloidosis (N=6) and in age- and body mass index (BMI)-matched controls (N=6). Subjects were advised to adhere to a 4-hour fasting period prior to the exams at fixed hours in the evening. Cine steady-state free precession images in standard short- and long-axis view were acquired for the assessment of global function and dimensions (Fig.1). Additional SSFP images in short axis and 4-chamber view were obtained to plan the location of the spectroscopic voxel. After shimming in breath hold, voxels of 8ml (4x2x1cm3) were placed in the interventricular septum (IVS) and spectra were recorded using a PRESS (point-resolved spectroscopy) sequence in systole (Fig.2). Spectroscopic data acquisition was double-triggered using ECG triggering and pencil beam navigator-based respiratory gating on the liver. Water suppression (WS) was achieved by frequency selective excitation. A total of 96 averages with water suppression (WS) and 16 averages without WS was recorded. Late gadolinium enhancement (LGE) imaging with a 3D gradient-spoiled fast field-echo sequence was performed to quantify fibrosis. Spectroscopy data were first reconstructed in MATLAB using a customized reconstruction pipeline implemented in ReconFrame (GyroTools LLC, Zurich, Switzerland) (Fig.1). Signal intensities were obtained by time-domain fitting the spectra in jMRUI/AMARES (version 5.2) (6). TG resonances were fitted at 0.9 and 1.3 ppm, the unsuppressed water at 4.7 ppm (Fig.2). Data were corrected for T1- and T2-relaxation effects and the triglyceride-to-water ratio (TG/W) was calculated (Fig.1). Additional baseline CMR imaging parameters included: 1) left-ventricular ejection fraction (LV-EF), 2) left ventricular mass indexed per body surface are (LVMi), 3) IVS thickness and 4) extent of LGE as % of the myocardial wall.Results
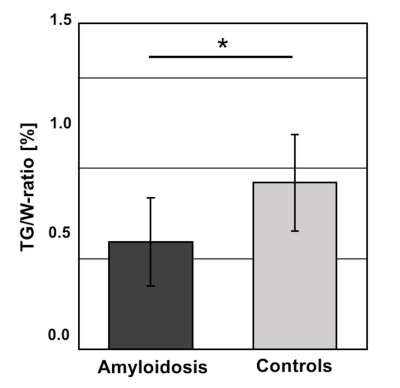
Patients with ATTR- or AL-amyloidosis had a mean age of 65.7±10.9years, BMI: 23.4±1.0kg/m2 while controls had a mean age of 64.5±6.5years, and a BMI: 22.9±3.9kg/m2. There were significant differences in patients vs. controls for IVS (20.7±3.6 vs. 8.2±1.0mm, p<0.01), LVMi (87.9±21.8 vs. 59.0±9.4 g/m2, p<0.01) and the amount of fibrosis (45.3±30.0% in amyloidosis). Significantly lower TG/W was measured in amyloidosis vs. controls (0.59±0.24 vs. 0.92±0.27%, p=0.05) (Fig.3). A tendency was seen for the amyloidosis group compared to plasma TG levels and BMI (R=-0.6, both). In addition, there was a negative tendency between increasing blood levels of N-terminal prohormone of brain natriuretic peptide (NT-proBNP) and TG/W (R=-0.63). ROC analysis revealed an area under the curve of 0.83 for TG/W to differentiate between amyloidosis and controls (Sensitivity 86%, Specificity 67%).Discussion
Proton spectroscopy revealed decreased myocardial TG/W in cardiac amyloidosis relative to age- and BMI-matched controls. Results of TG/W in controls agree with findings of previous MRS studies of aged hearts, although standard deviations are high in reported literature (7,8). In addition, reduced TG/W is in line with data reported in previous studies demonstrating reduced fatty acid utilization in advanced stages of heart failure (HF) and in hypertrophic cardiomyopathy (4,9). Correlation of NT-proBNP as a marker of HF and correlation to BMI and blood TG remained less pronounced in contrast to suggested positive associations in previous studies (7,10). This may suggest other mechanisms involving myocardial TG metabolism including known lipid association to amyloid proteins (11,12). Further differentiation, e.g. between different forms of amyloidosis, may be achieved with increasing subject numbers.Conclusion
Proton spectroscopy may provide additional information in patients with amyloidosis along with standard imaging readouts. It is hypothesized that two mechanisms may influence myocardial TG including a reduction of TG during stages of advanced heart failure as well as lipid association of tissue amyloid depositions.Acknowledgements
No acknowledgment found.References
1. Driesen RI vanden, Slaughter RE, Strugnell WE. MR Findings in Cardiac Amyloidosis. Am J Roentgenol. 2006;186(6):1682–1685.
2. Quarta CC, Kruger JL, Falk RH. Cardiac Amyloidosis. Circulation. 2012;126(12):e178-e182.
3. Dungu JN, Valencia O, Pinney JH, et al. CMR-Based Differentiation of AL and ATTR Cardiac Amyloidosis. JACC Cardiovasc Imaging. 2014;7(2):133-142.
4. Neubauer S. The Failing Heart — An Engine Out of Fuel. N Engl J Med. 2007;356(11):1140–1151.
5. Maleszewski JJ. Cardiac amyloidosis: pathology, nomenclature, and typing. Cardiovasc Pathol. 2015;24(6):343–350.
6. Vanhamme L, Boogaart A van den, Huffel S Van. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129(1):35–43.
7. Sarma S, Carrick-Ranson G, Fujimoto N, et al. Effects of age and aerobic fitness on myocardial lipid content. Circ Cardiovasc Imaging. 2013;6(6):1048–1055.
8. Meer RW van der, Rijzewijk LJ, Diamant M, et al. The ageing male heart: myocardial triglyceride content as independent predictor of diastolic function. Eur Heart J. 2008;29(12):1516–1522.
9. Nakae I, Mitsunami K, Yoshino T, et al. Clinical features of myocardial triglyceride in different types of cardiomyopathy assessed by proton magnetic resonance spectroscopy: comparison with myocardial creatine. J Card Fail. 2010;16(10):812–822.
10. Gaborit B, Kober F, Jacquier A, et al. Assessment of epicardial fat volume and myocardial triglyceride content in severely obese subjects: relationship to metabolic profile, cardiac function and visceral fat. Int J Obes. 2012;36(3):422–430.
11. Ami D, Lavatelli F, Rognoni P, et al. In situ characterization of protein aggregates in human tissues affected by light chain amyloidosis: a FTIR microspectroscopy study. Sci Rep. 2016;6(1):29096.
12. Miyahara H, Sawashita J, Ishikawa E, et al. Comprehensive proteomic profiles of mouse AApoAII amyloid fibrils provide insights into the involvement of lipoproteins in the pathology of amyloidosis. J Proteomics. 2017; doi: 10.1016/j.jprot.2017.10.003.
Figures
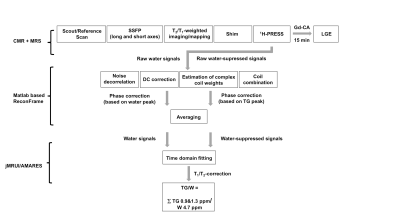
Figure 1: Flow chart of the exam protocol for CMR and MRS imaging and reconstruction pipeline used for MRS data.
SSFP: steady state free precession; PRESS: point-resolved spectroscopy; Gd-CA: gadolinium based contrast agent; LGE: late gadolinium enhancement; TG: triglycerides.