4758
MRI-Based Stress Analyses of Abdominal Aortic Aneurysms With Resolved Intraluminal Thrombus Heterogeneity1Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States
Synopsis
Computational stress analyses of abdominal aortic aneurysms (AAA) are of great interest for individual aneurysm rupture risk assessment. The vast majority of patient-specific stress analyses are based on features seen at computed tomography, which is incapable of resolving material heterogeneity within intraluminal thrombus. Using T1-weighted black blood MRI, we imaged and explicitly modeled MRI-discerned intraluminal thrombus heterogeneity in multiple AAA stress analyses. Results demonstrate a limited effect of thrombus heterogeneity on the predicted vessel wall stresses, but suggest a possible role for MRI to inform thrombus material stiffness assignment in stress computations.
Introduction
Abdominal aortic aneurysms (AAA) are common, and their rupture is often fatal. Much work has focused on assessing rupture risk for individual patients using finite element wall stress estimations incorporating aneurysm features from medical imaging, principally computed tomography (CT). CT cannot resolve the material heterogeneity of the intraluminal thrombus (ILT) common in larger aneurysms, and the disparate stiffnesses of distinct thrombus layers, known from specimen testing, have not been incorporated in a patient-specific fashion. Using a T1-weighted black-blood fast spin echo acquisition as part of an MRI protocol for comprehensive AAA evaluation1, we delineate ILT layers in 4 AAA patients and study the effects of explicitly modeling ILT heterogeneity in stress analyses.Methods
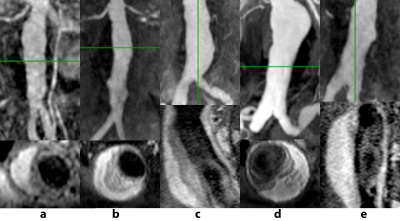
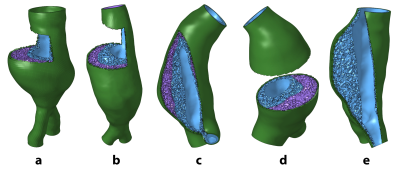
Four AAA patients (cases a-d) undergoing MRI surveillance in an IRB-approved study were selected for having significant ILT burden with two distinct layers on T1-weighted black-blood imaging: low signal in thrombus adjacent to the lumen and high signal closer to the vessel wall (Figure 1, a-d). A 5th case (e) was selected for uniformly high signal intensity throughout the ILT (Figure 1, e). The geometric boundaries of different ILT layers were segmented manually from black blood imaging, while CE-MRA and post-contrast VIBE imaging were used to segment the flow lumen and vessel wall, resulting in a set of 3-dimensional surfaces that served as the basis for computational mesh generation. Following a sensitivity analysis, the AAA wall was meshed with a linear hexahedra dominant scheme. ILT was meshed with hybrid linear tetrahedra and boundary pyramid elements (Figure 2). The vessel wall and ILT were considered incompressible, with hyperelastic constitutive relations selected from the literature. The stiffer luminal ILT was represented by a polynomial strain energy density function derived from Di Martino and Vorp2, and the weaker abluminal ILT was represented as an Ogden-type material as derived by Gasser et al3. The specific parameters describing ILT types were motivated by the work of Riveros et al4, wherein the effects of different ILT stiffness were considered for uniform thrombus. For cases a-d, ILT was modeled as heterogeneous, uniformly stiff, and uniformly weak. Case e with uniform ILT signal intensity considered only stiff or weak thrombus. A fixed-point iterative technique was used to estimate the unloaded geometry of each AAA, with subsequent pressurization to 120 mmHg. Vessel displacements at the inlet and outlets were constrained to be radially oriented. Von Mises stresses in the AAA were computed using the ABAQUS solver.Results
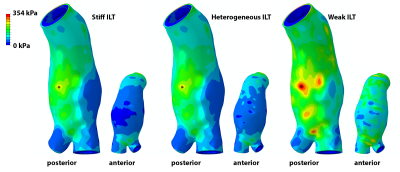
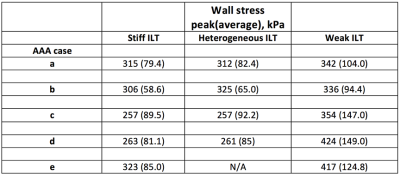
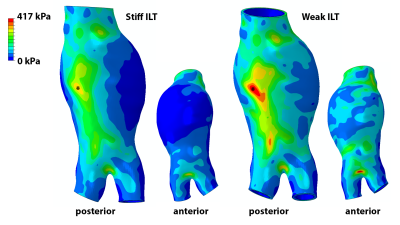
Example AAA wall stress distributions are shown for case c in Figure 3 and listed for all cases in Figure 4. Explicit modeling of ILT heterogeneity based on MRI had virtually no effect on peak wall stress compared to the results of models assuming uniformly stiff ILT. Peak stress location was unchanged for each case, while the stress magnitude differed by only 6% for case b, and was within 1% for cases a, c, and d. Average vessel wall stress increased minimally with the incorporation of ILT heterogeneity when compared to the uniformly stiff ILT models, reflecting modestly higher stresses in the most dilated aneurysm segments, where the adjacent weak thrombus layer is thickest. Peak wall stress magnitude increased by an average 27% (SD = 23%) when thrombus was considered uniformly weak, though peak stress location was not significantly changed in any case. As an example, case e is shown in Figure 5.Discussion
MRI has the unique capability to discern heterogeneity within intraluminal thrombus in AAAs, which has thus been considered homogeneous in the dominantly CT-based AAA stress analysis literature. Results for cases a-d demonstrate that for the combination of ILT material models considered, incorporation of MRI-discerned ILT heterogeneity may have little effect on aneurysm wall stress estimates. This is important, as segmentation and mesh generation become more complicated and time consuming each time an additional material boundary is represented in a patient-specific simulation. Eliminating complexity, when reasonable, can increase simulation throughput, which has traditionally been limited when considering patient-specific geometries. However, MRI may help inform the assignment of uniform ILT stiffness in simulations, as suggested by case e, potentially improving stress prediction accuracy. This hinges on verification that thrombus signal intensity is indeed correlated with mechanical stiffness, and work is ongoing to examine this relationship. As this work considered a small variety of ILT distributions, further investigation is continuing over a broader set of geometries and ILT material models.Conclusion
MRI-discerned intraluminal thrombus heterogeneity may have limited influence in computational AAA wall stress analyses, although further investigation over a larger set of material models is warranted.Acknowledgements
Work supported by the National Institutes of Health (NIBIB) T32 Training Grant, T32EB001631References
1. Zhu, C., et al., Non-contrast 3D black blood MRI for abdominal aortic aneurysm surveillance: comparison with CT angiography. Eur Radiol, 2017. 27(5): p. 1787-1794.
2. Di Martino, E.S. and D.A. Vorp, Effect of variation in intraluminal thrombus constitutive properties on abdominal aortic aneurysm wall stress. Ann Biomed Eng, 2003. 31(7): p. 804-9.
3. Gasser, T.C., et al., Failure properties of intraluminal thrombus in abdominal aortic aneurysm under static and pulsating mechanical loads. J Vasc Surg, 2008. 48(1): p. 179-88.
4. Riveros, F., et al., On the impact of intraluminal thrombus mechanical behavior in AAA passive mechanics. Ann Biomed Eng, 2015. 43(9): p. 2253-64.
Figures