4757
Significant Improvement of Blood Suppression in Cardiac Dark-Blood T1-TSE Imaging By Combining the T2-TSE Blood Nulling Strategy with Dummy Acquisitions for Myocardial T1-Weighting – A Comparison of Conventional and Novel TSE Techniques for Blood Signal Suppression and Overall Image Quality at 3T1MR R&D Collaborations, Siemens Healthineers, Malvern, PA, United States, 2Duke Cardiovascular MR Center, Duke University, Durham, NC, United States
Synopsis
Cardiac dark-blood (DB) T1-turbo-spin echo (TSE) imaging frequently suffers from poor blood suppression due to its short effective TR required for good myocardial T1-weighting. We present a novel DB T1-TSE sequence that simultaneously applies a long effective TR as in T2-TSE to blood and a short effective TR as in conventional T1-TSE to myocardium, by means of dummy readouts. We ran conventional T1-TSE, T2-TSE, and novel T1-TSE sequences in 53 patients and scored the images for DB performance and overall image quality. Quality and blood suppression were drastically improved by novel T1-TSE.
INTRODUCTION
High spatial resolution dark-blood (DB) turbo spin-echo (TSE) imaging for T1- and T2-contrast is important for assessing cardiac morphology and characterizing masses. T1- and T2-mapping1,2 are helpful in examining diffuse myocardial processes, but suboptimal for detecting subendocardial abnormalities as they are single shot techniques with intrinsically limited spatial resolution. Blood suppression in DB T2-TSE images usually works very well, because the long effective TR of typically 2 RR intervals allows significant T1-recovery of blood and thus its proper nulling in diastole. Conversely, conventional T1 (CONV-T1) TSE requires a short effective TR of 1 RR interval for sufficient myocardial T1-weighting (T1W), but at most physiologic heart-rates (HR) such short TR unfortunately prevents adequate blood suppression, particularly at 3T. Additionally, long echo-spacing (ES) renders conventional TSE readouts motion-sensitive causing myocardial signal-dropout and blurring. In combination these issues frequently lead to poor image quality (IQ) of most CONV-T1 TSE images. To address these problems we created a novel T1 (NOV-T1) TSE acquisition-scheme combining longer effective blood-TR for complete blood suppression as in T2-TSE with shorter myocardium-TR for optimal myocardial T1W as in CONV-T1 TSE. We also designed new readout pulses to shorten ES for reduced motion-sensitivity, while simultaneously achieving a higher time-bandwidth product (BWTP) for sharper slice profiles.
METHODS
Figure 1 shows CONV-T1 (1a), T2 (1b), and a NOV-T1 (1c) DB-TSE sequences with typical timing. CONV-T1 plays a DB preparation (DBP) and readout every RR. T2-TSE does so every other RR. NOV-T1 TSE applies a DBP every other RR (blood-TR=2RR) as in T2-TSE, but keeps tissue T1W commensurate with TR=RR by alternating true with dummy readouts. Optimal blood-TR was determined by a devised algorithm, see caption of Figure 1. For NOV-T1 and T2-TSE, Hanning-filtered sinc pulses with a higher BWTP than in CONV-T1 were truncated below 5% of peak-amplitude to reduce pulse-duration (excitation BWTP 1.6, 732µs, refocusing BWTP 2.3, 1300µs) while keeping their area essentially constant. 6 TSE images of the same spatial and temporal resolution, 2 CONV-T1, 2 T2, and 2 NOV-T1, were acquired at 2 locations per patient on a MAGNETOM Verio 3T clinical MR scanner (Siemens Healthineers). In all images, blood signal, myocardium signal, blood signal-to-noise-ratio (SNR), myocardium SNR and blood-to-myocardium-signal-ratio (BMR) were measured. Furthermore, the NOV-T1 and CONV-T1 TSE images were graded for IQ (4=excellent, 3=good, 2=poor, 1=non-diagnostic) by experienced readers. For all comparisons paired t-tests were used.
RESULTS
Patients (25F, 28M) had an RR of 916±151ms (MEAN±STDEV). CONV-T1 used blood-TR=RR. NOV-T1 and T2 employed blood-TR=2RR by algorithm in all but 1 patient. CONV-T1 images required 8 echo trains (ET) in 8 RRs, NOV-T1 and T2 6 ETs in 12 RRs. NOV-T1 acquisition time increased only 50% despite 100% longer blood-TR, due to faster readout of NOV-T1 (21 echoes at 3.94ms ES) relative to CONV-T1 (16 at 5.27ms ES). NOV-T1 blood signal was significantly more suppressed than in CONV-T1 (37.06 ±0.14 vs 104.23±0.27, p<0.0001). T2 blood signal was even lower than in NOV-T1 (19.21 ±0.06 vs 37.06 ±0.14, p<0.0001). T2- and NOV-T1 completely nulled blood magnetization in the simulated recovery curves (Figure 1d), whereas CONV-T1 did not. NOV-T1 provided significantly better IQ (MEAN±SEM 3.58±0.07 vs 2.73±0.09, p<0.001) and darker blood by SNR (7.26±0.68 vs 14.65±1.09, p<0.0001) and BMR (0.175±0.09 vs 0.35±0.12, p<0.0001) than CONV-T1. Myocardium-SNR was identical between CONV-T1 and NOV-T1 (44.28±4.20 vs 44.83±4.44). Figure 2 shows that DB, sharpness, and overall IQ were drastically improved from CONV-T1 to NOV-T1 and that DB quality was excellent for both T2 and NOV-T1.
DISCUSSION
Applying the blood nulling strategy of DB T2-TSE to NOV-T1 is likely responsible for the dramatic improvement in blood suppression. As a consequence, the haze across cavity and myocardium present in nearly all CONV-T1 images was not present in the corresponding T2 and NOV-T1 images. We attribute the even darker blood in the T2-TSE images compared to NOV-T1 to the known dephasing effect of the TSE readout3 increasing with effective TE. The crisper image appearance (Fig. 2) of NOV-T1 and T2-TSE images compared to CONV-T1 is likely a result of the better slice profile of the new readout pulses and the improved motion robustness by shorter ES.
CONCLUSION
NOV-T1 TSE allows clinical image acquisition with excellent diagnostic quality, high spatial resolution, and optimal DB performance at any clinically-relevant HR, without affecting myocardium-SNR and T1W. T2-TSE imaging already has excellent blood suppression and appears to benefit from the described shorter readout pulses.
Acknowledgements
No acknowledgement found.References
1. Messroghli, D. R., Radjenovic, A., Kozerke, S., et al. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004 Jul;52(1):141-6.
2. Giri, S., Chung, Y.-C., Merchant, A., et al. T2 quantification for improved detection of myocardial edema. JCMR 2009 Dec;11: 56-60
3. Berkowitz, S.J., Macedo, R., Malayeri, A. A., et al. Axial Black Blood Turbo Spin Echo Imaging of the Right Ventricle. Magn Reson Med. 2009;61(2):307-14.
Figures
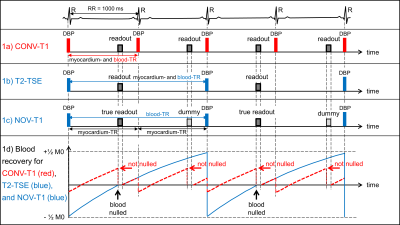
Figure 1: a) CONV-T1 TSE with DB preparation (DBP) and readout every RR (blood-TR=RR, red). b) T2-TSE with DBP and readout every other heartbeat (blood-TR=2RR, blue) as determined by devised algorithm using RR=1000ms: blood-TR=RR for RR>1250ms, 2RR for 600ms≤RR≤1250ms, and 3RR for RR<600ms. c) NOV-T1 with DBP every other heartbeat (using same algorithm), but true and dummy readouts alternating creating an effective myocardium-TR=RR. d) Blood T1 recovery curves (T1 =1900 ms at 3T) for CONV-T1 (red) and T2-TSE/NOV-T1 (blue). Blood is nulled at readout for T2 and NOV-T1, but not for CONV-T1.
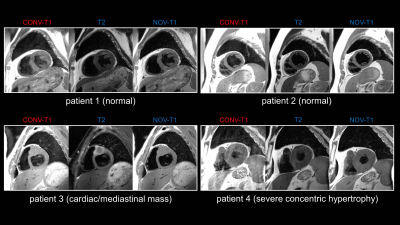
Figure 2: Typical T1- and T2-TSE patient images acquired with CONV-T1, T2- and NOV-T1 TSE sequences showing better blood suppression, image sharpness, and overall image quality of NOV-T1 and T2-TSE compared to CONV-T1. Note the haze across the heart for CONV-T1 due to poor blood nulling, which is removed in T2-TSE and NOV-T1 images. Typical parameters were FOV 340x255mm, resolution 1.4x1.4mm, slice thickness 5mm. CONV-T1 used TE 11ms and turbo factor (TF) 16, NOV-T1 TE 12ms and TF 21, T2-TSE TE 67ms and TF 21. Temporal resolution was matched to ≈84ms. Acquisition took 8 (CONV-T1) and 12 (NOV-T1/T2-TSE) heartbeats.