4754
Imaging lower extremity vein thrombosis using a 3D FSE with novel modulated flip angle scheme and random k-space undersampling1Department of Radiology, Beijing Luhe Hospital, Capital Medical University, Beijing, China, 2Department of Vascular Surgery, Beijing Luhe Hospital, Capital Medical University, Beijing, China, 3Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University, Beijing, China, 4United Imaging Healthcare Co. Ltd, Shanghai, China
Synopsis
Accurate detection of lower extremity vein thrombosis (LEVT) and assessment of its stage are crucial for treatment decision-making. Challenges such as long scan time and requirement for dark-blood, exist in T2-weighted MR imaging of LEVT. A 3D FSE with novel modulated refocusing flip angles and random undersampling is introduced and applied to imaging of LEVT.
Introduction
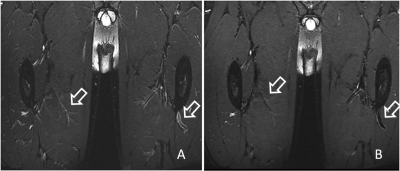
Lower extremity vein thrombosis (LEVT) is a common condition with high morbidity and disability rate.Lower extremity deep vein thrombosis (LEDVT) is a type of LEVT and the main cause of fatal pulmonary embolism. Accurate detection of LEVT and assessment of its stage are therefore crucial for treatment decision-making. To meet the demand for 3-dimensionalhigh resolution, reliable suppression of blood signal and acceptable scan time in T2-weighted imaging of LEVT,a novel 3D FSE sequence using modulated refocusing flip angles and random k-space undersampling(referred to as MATRIX) was used. Unlike in Mugler and Busse‘s methods 1,2, which calculate the variable refocusing flip angles according to a prescribed signal evolution scheme, MATRIX directly chooses an optimal modulation of flip angles from a pool of candidates, which are generated through a formula determined by some flexible constraints, such as smoothness of the signal evolution, desired tissue contrast and sensitivity to flow and etc 3. To achieve a reliable suppression of flowing blood signal, motion sensitizing gradients were implemented in MATRIX. Suppression of blood can be further improved by fine-tuning the modulation of flip angles (see Figure 1). Random k-space undersampling with iterative reconstruction was implemented to achieve high acceleration in thin slab 3D acquisition without suffering from residual aliasing artifacts in typical parallel imaging 4. The feasibility of the proposed MATRIX was evaluated in both healthy volunteers and patients with suspected LEVT by comparing to T1 MPARGE imaging.Methods
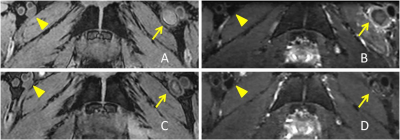
A total of 11 cases with acute LEVT detected by ultrasound, with D-dimer> 500μg/L on admission and clinical manifestation within 15 days, were prospectively enrolled in this study. All patients underwent 3.0T MR scans(uMR 770, UIH, Shanghai, China). Both 3D T2-weighted MATRIX and T1-weighted MPRAGE were performed in every examination with an isotropic resolution of 1 mm. The acquisition parameters of MATRIX: TR/TE=2000 ms/335 ms, ETL=100, Acceleration factor 3.0. Four patients received follow-up scans after 1 month.The scan covered from inferior vena cava to knee, or knee to ankle, or both two ranges above. The reconstructed images were transected with the baseline parallel to the upper edge of the public symphysis or bilateral tibial plateau with a thickness of 0.6 mm. In the multi-planar reconstruction, bilateral lower limbs were observed, and three consecutive slices with thrombus were selected. The thrombus area and signal intensity were measured on both T1WI and T2WI by two independent observers (Figure 2).The diagnostic agreement of thrombus detection between T2WI and T1WI was conducted using Cohen κ test. The agreement between T1WI and T2WI in the measurement of thrombus area at the segmental level was performed by Bland-Altman analysis.Results
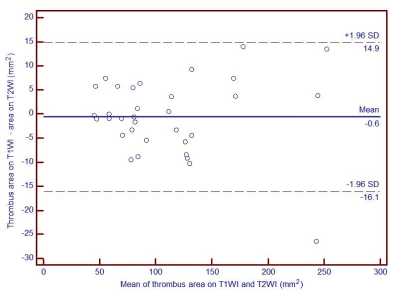
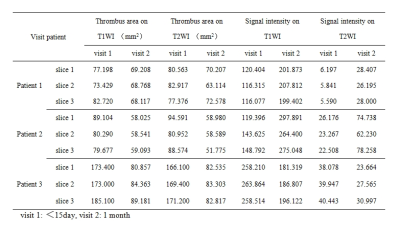
Thirty segments (24 segments of deep vein, 6 segments of superficial vein) were included in the study. There was a good agreement between the T1WI and T2WI in the detectionof LEVT ( κ= 0.933, p = 0.000)and LEDVT (κ= 1.000, p = 0.000).In contrast, there was a poor agreement (κ= 0.667, p = 0.083) in detection of lower extremity superficial vein thrombosis. Bland-Altman chart also showed strong agreement between T1WI and T2WI in the measurement of thrombus area in LEDVT (Figure 3). Among the 4 LEDVT patients with follow-up scans, thrombus disappeared in 1 patient who underwent a thrombectomy, but was still present in the other 3 patients.The size of the thrombus was decreased remarkably on both T1WI and T2WI at the follow-ups. The signal intensity of thrombus increased in 2 patients while decreased in 1 patient on both T1WI and T2WI (Table 1).Discussion and Conclusion
3D isotropic MATRIX imaging provides sufficient coverage of interested regions with acceptable scan time, and its arbitrary multi-planar reconstruction facilitates the visualization and detection of suspected thrombus.This preliminary study shows the results of MATRIX are highly consistent with that of MPRAGE in the detection of LEVT. Furthermore, the combination of MATRIX and MPRAGE may help to assess the stage of thrombus with great confidence, as reported in an early study using 2D imaging5. In our follow-up scans (see Table 1), two patients showed the reduction of thrombus area and increase of signal intensity between the two consecutive examinations, which can be interpreted as the thrombus was transiting from acute to medium stage. It is interestingly noticed that the signal intensity of thrombus in one patient decreased, which means a transition of thrombus stage from medium to old. This observation may infer that a subjective assessment of the thrombus stage is not reliable on the admission. In the future, a longitudinal study with a larger group of samples will be conducted with MATRIX technique in staging of LDVT.Acknowledgements
No acknowledgement found.References
1. J.P.Mugler,III, H. Meyer, B. Kiefer, Practical Implementation of Optimized Tissue-Specific Prescribed Signal Evolutions for Improved Turbo-Spin-Echo Imaging. ISMRM 2003, p203.
2. Reed F. Busse,Anja C.S. Brau, Anthony Vu et al, Effects of Refocusing Flip Angle Modulation and ViewOrdering in 3D Fast Spin Echo. MRM 2008; 60:640-649
3. Li GB, WANG CH.System and method for flip angle determination in magnetic resonance imaging.US patent:20170212197 A1, August 21, 2015.
4. Klaas P. Pruessmann, MarkusWeiger, Markus B. Scheideggeret al, SENSE: Sensitivity Encoding for Fast MRI. MRM 1999; 42: 952–962.
5. Corti R, Osende JI, Fayad ZA, et al. In vivo noninvasive detection and age definition of arterial thrombus by MRI.J Am CollCardiol. 2002 Apr 17;39(8):1366-73.
Figures