4752
Accelerated Coronary 4D-Flow MRI: towards noninvasive functional assessment of stable coronary artery disease1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Radiological Sciences, University of California, Los Angeles, Los Angeles, CA, United States, 3Siemens Healthcare R&D, Los Angeles, CA, United States
Synopsis
In patients with suspected coronary artery disease (CAD), invasive catheterization is commonly used to determine the need for coronary revascularization. However, recent studies have shown that >50% of patients who undergo invasive catheterization have non-significant coronary lesions, hence the procedure was unnecessary. Recent work using 4D-Flow and the Navier-Stokes equations has shown promise for the noninvasive assessment of
Introduction
In the current clinical management of patients with suspected coronary artery disease (CAD), invasive catheterization procedures such as invasive coronary angiography (ICA) and/or fractional flow reserve (FFR) are used as the final diagnostic work-up to determine the need for coronary revascularization1. However, recent studies have shown poor patient selection for invasive catheterization where more than 50% of patients who undergo invasive procedures were found to have non-obstructive (ICA<50% degree stenosis) or functionally non-significant (FFR>0.80) coronary lesions2-4. This suggests that improvements in noninvasive diagnostic techniques are needed to better classify patients who are most suited for invasive catheterization and revascularization. A recent noninvasive technique using phase-contrast MRI and the Navier-Stokes equations to estimate the pressure gradient across a coronary stenosis has shown promise in identifying patients with functionally nonsignificant stenosis5. However, the method requires long scan times (30-40 min), which hinders the translation of the method into a clinical setting. This work aims to develop a 4D-Flow technique for the coronary arteries using golden-angle stack-of-stars (SOS) acquisition with compressed sensing reconstruction to achieve 2-3x accelerated imaging. The goal was to develop a more clinically feasible noninvasive pressure gradient measurement method for the assessment of CAD.Methods
A golden-angle SOS 4D-Flow sequence with ECG-triggering to diastole and navigator-gating to end-expiration was implemented for coronary imaging on a 3T MR scanner (Skyra, Siemens). Compressed sensing reconstruction was performed individually for each velocity encoding direction (reference, x, y, and z) and each cardiac phase by solving the following optimization problem6 $$$d=arg min \{||FSd - m||_2^2+ λ||Td||_1\}$$$, where $$$d$$$ contains the image, $$$F$$$ is the non-uniform fast Fourier Transform operator, $$$S$$$ is the coil sensitivity operator, $$$m$$$ is the measured k-space data, $$$λ$$$ is the regularization term, and $$$T$$$ is the sparsity transform where a 4-level 3D Daubechies-4 wavelet transform was used. Coil sensitivity maps were computed using the reference velocity encoding and applied to the other encoding directions.
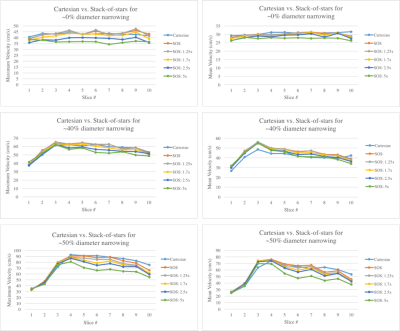
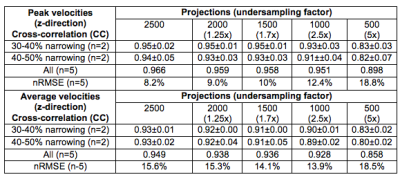
In vitro: SOS and Cartesian were first compared in five stenotic flow phantoms (constant volume velocity=250mL/min; reference diameter=4.8mm) with diameter narrowing (stenosis) ranging between 0%-50%. Shared imaging parameters were: FA=15°; spatial resolution=0.5x0.5x3.2mm3; Partition=10; slice oversampling=25%; FOV=220mm; VENC=70-110cm/s, depending on degree of narrowing. For SOS, a total of 2500 projections per flow encoding direction were obtained corresponding to ~22min acquisition time with simulated 60-bpm ECG-triggering, matched to similar scan time as the Cartesian 4D-flow acquisition with a parallel imaging acceleration of 2x. To evaluate the feasibility of scan time reduction in the SOS method, retrospective k-space undersampling was achieved by discarding the sampled data after 2000, 1500, 1000, and 500 projections from a total of 2500 projections. Cross-correlation and root-mean-square error (RMSE) in the peak and average velocities across all imaging slices was performed for a range of SOS projections versus Cartesian, respectively.
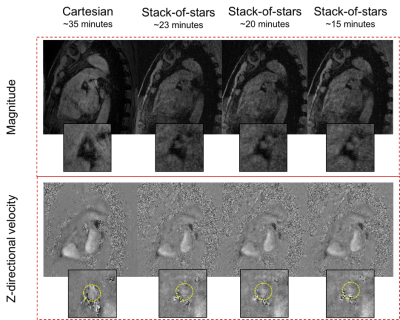
In vivo: SOS and Cartesian 4D-flow sequences were then tested in three healthy subjects (age: 40.7±17.9yrs). Similar imaging parameters were used in in-vitro studies except for: spatial resolution = 0.6x0.6x3.2mm3; Partitions=8; VENC=40-45cm/s in the proximal to middle segment of the left coronary artery. Due to limited scan time, a total of 1200 projections (~1.5x undersampling compared to Cartesian) were collected per velocity encoding, per cardiac phase at the time of the scan. Retrospective k-space undersampling was achieved by discarding the sampled data after 1000 and 800 projections from a total of 1200 projections, equivalent to 1.8x and 2.3x undersampling, respectively.
Results
In vitro: When comparing to Cartesian acquisitions, similar peak velocities across each imaging slice were observed over a range of SOS undersampling factors (Figure 1) and good cross-correlation of up to 2.5x undersampling, 0.95 and 0.93, was preserved in the peak and average velocities, respectively, as SOS projection number decreased (Table 1).
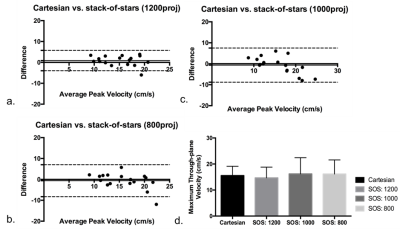
In vivo: Total average Cartesian scan time was 35.46±0.3minutes. For SOS, 1.5x, 1.8x, and 2.3x undersampling factor, equivalent to approximately 23, 20, and 15 minutes scan time, respectively, using Cartesian as a reference. Good image quality was observed for various SOS underdamping factors as shown in figure 2. Average maximum through-plane velocity in all healthy subjects was 15.6±3.6 cm/s, 14.7±4.1 cm/s, 16.2±6.2 cm/s and 16.2±5.4 cm/s for Cartesian, and SOS with 1200, 1000, and 800 projections, respectively. Figure 3 shows the Bland-Altman of the maximum through-plane velocities between various SOS undersampling factors and Cartesian acquisition.
Conclusion
Our preliminary results showed the feasibility of SOS 4D-Flow measurement in the coronary arteries. It has the potential to reduce scan time (2-3x) as compared to conventional Cartesian imaging. In vivo studies involving patients with coronary artery disease are underway to evaluate the performance of the method.Acknowledgements
No acknowledgement found.References
1. Fihn S, Blankenship J, Alexander K, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2014; CIR-0000000000000095.
2. Patel MR, Peterson ED, Dai D, et al. Low Diagnostic Yield of Elective Coronary Angiography. N. Engl. J. Med. 2010;362:886–895. doi: 10.1056/NEJMoa0907272.
3. Patel MR, Dai D, Hernandez AF, et al. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice. 2014;167:846–852.e2. doi: 10.1016/j.ahj.2014.03.001
4. Tonino PAL, Fearon WF, De Bruyne B, et al. Angiographic Versus Functional Severity of Coronary Artery Stenoses in the FAME Study. JACC 2010;55:2816–2821. doi: 10.1016/j.jacc.2009.11.096
5. Deng Z, Fan Z, Lee S-E, et al. Noninvasive measurement of pressure gradient across a coronary stenosis using phase contrast (PC)-MRI: A feasibility study. Magn Reson Med 2016;77:529–537. doi: 10.1002/mrm.26579
6. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med 2007;58:1182–1195. doi: 10.1002/mrm.21391
Figures