4751
Simultaneous 3D whole-heart bright-blood and black blood imaging for cardiovascular anatomy and wall assessment with interleaved T2prep-IR1King's College London, London, United Kingdom, 2Siemens Healthcare, London, United Kingdom
Synopsis
Visualization of cardiovascular anatomy is important for diagnosis, risk stratification and planning of interventional procedures both in patients with congenital and non-congenital heart disease. This study proposes a novel free-breathing 3D whole-heart flow-independent approach for bright-blood visualization of coronary lumen and cardiac structures and for black-blood delineation of aortic, atrial, and coronary artery walls. The use of image-based navigation and non-rigid respiratory motion correction allows for 100% scan efficiency, predictable scan time and improved image sharpness.
Introduction
Visualization of cardiovascular anatomy is important for diagnosis, risk stratification and planning of interventional procedures both in patients with congenital and non-congenital heart disease. MRI is a powerful tool for the assessment of cardiac anatomy including bright-blood visualization of coronary lumen and cardiac structures and black-blood delineation of aortic, atrial and coronary artery walls. Conventional 2D double inversion recovery1 (DIR) black-blood vessel imaging techniques have been successfully used to visualize vessel wall of aorta, carotid and coronary arteries. However, DIR techniques provide limited coverage of the heart and are flow-dependent. A 3D flow-independent approach for simultaneous coronary lumen and vessel wall visualization (iT2prep) was proposed recently2; it is based on an interleaved acquisition and subtraction of data with and without T2-preparation. iT2prep incorporates 2D-image-based navigators3 (iNAV) for 100% scan efficiency and non-rigid4 respiratory motion correction. However, this technique requires subject-dependent weighted subtraction to completely null arterial blood signal. Here we propose an interleaved T2prep-IR approach (iT2prep-IR) that: a) improves vessel wall-to-blood contrast and b) removes the need of weighted subtraction. Additionally, the non-rigid motion correction framework is optimized for the proposed technique.Methods
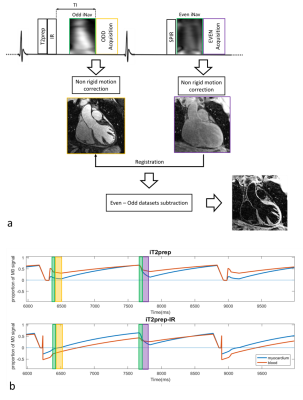
The proposed iT2prep-IR bright-and-black-blood sequence is based on the acquisition and direct subtraction of two interleaved 3D-whole-heart image datasets acquired as shown in Fig.1a. A T2Prep-IR module is applied prior to data acquisition in odd heartbeats, allowing bright-blood visualization of cardiac anatomy, whereas even datasets are obtained without use of preparation pulses. Low-resolution 2D-iNAVs are acquired in each heartbeat to estimate superior-inferior (SI) and right-left (RL) translational respiratory motion without any data rejection. Non-rigid motion compensation is performed independently for the two datasets and subsequent non-rigid image alignment is performed prior to datasets subtraction. Sequence simulations based on EPG formalism5 were performed for the proposed and iT2prep techniques (Fig1b) for heart rate (HR) of 45bpm and 70bpm.
Acquisition: Four healthy subjects were scanned under free-breathing on a 1.5T system (Siemens Magnetom Aera) with the proposed and iT2prep approaches. The iT2prep-IR prototype sequence was acquired with an ECG-triggered 3D Cartesian b-SSFP imaging sequence (resolution=1x1x2mm, FOV=320x320x144mm, TR/TE=3.6/1.6ms, flip-angle=90deg, TI=110ms, T2Prep duration=40ms, 14 startup-echoes for iNAV). Odd heartbeat acquisitions included a SPIR pulse for fat saturation, while a STIR-like fat suppression was employed in even heartbeats. iT2prep acquisitions were performed with T2prep duration=70ms (odd heartbeats) and matching imaging parameters for comparison purposes.
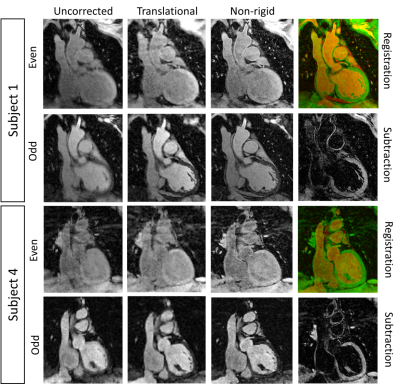
Reconstruction: For both iT2prep and iT2prep-IR acquisitions, odd and even low-resolution iNAVs were acquired to estimate SI and RL translational respiratory motion and to perform data binning. Soft-gated iterative SENSE bin reconstruction was performed to estimate non-rigid motion fields (from inter-bin registration) which subsequently were incorporated in the reconstruction4. Additional non-rigid image alignment was performed between motion corrected odd and even datasets before subtraction to obtain the black-blood volume.
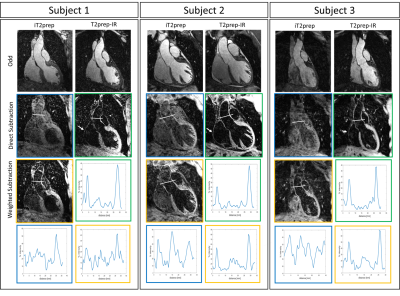
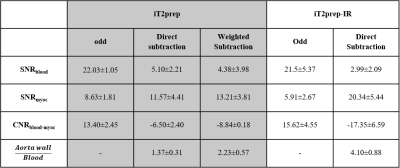
Data analysis: Signal-to-noise and contrast-to-noise ratio of blood and myocardium (SNRblood, SNRmyoc, CNRmyoc-blood) were computed for both iT2prep-IR and iT2prep acquisitions. Aortic vessel wall thickness obtained with the two sequences was quantified and compared by computing intensity profiles across the aorta.
Results
Simulations: Sequence simulations of the proposed iT2Prep-IR approach (Fig.1b) show almost identical absolute blood magnetization in odd and even heartbeats for both low (0.26 and 0.30) and high (0.17 and 0.21) HR, leading to complete blood nulling after subtraction and better myocardium-blood contrast compared to iT2prep (myocardium-blood-ratio of 6.57 and 2.95 respectively for 45bpm and 4.97 and 3.09 for 70bpm).
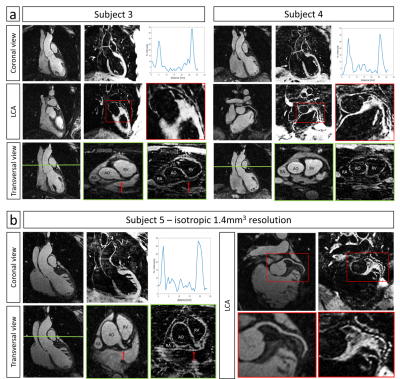
Healthy subjects: Improved nulling of the blood signal with iT2Prep-IR in comparison to both direct and weighted iT2prep subtraction was obtained without need for weighted subtraction (Fig.2). iT2prep-IR sequence generated higher CNRmyoc-blood in odd and subtracted datasets (Table1). Aortic vessel wall thickness showed a correlation of 0.88 between iT2prep and iT2prep-IR sequences. Additionally, intensity profiles across the aorta showed higher wall-to-blood ratio for the iT2prep-IR subtracted dataset compared to both direct and weighted iT2prep subtraction (Table1). The non-rigid motion correction framework improved image quality for both odd and even iT2prep-IR datasets compared to uncorrected and translational corrected images (Fig.3) leading to visualization of the left coronary artery (LCA), right ventricular wall and right atrium wall in the subtracted dataset (Fig.4a).
Conclusion
The proposed iT2prep-IR sequence showed promising results in terms of blood signal nulling, SNRmyoc in the odd dataset and CNRmyoc-blood in both bright-blood and black-blood (wall) imaging datasets without the need of weighted subtractions. Aortic, right ventricular, right atrium and proximal LCA wall were visible after non-rigid motion correction for all the acquired subjects. Future work will include acquisition of higher isotropic resolution images to improve coronary vessel wall depiction by minimizing partial volume artefacts as suggest by Fig.4b.Acknowledgements
This work is funded by the King’s College London & Imperial College London EPSRC Centre for Doctoral Training in Medical Imaging (EP/L015226/1) and Siemens HealthcareReferences
1) Botnar RM, Kim WY, Börnert P, Stuber M, Spuentrup E, Manning WJ. 3D coronary vessel wall imaging utilizing a local inversion technique with spiral image acquisition. Magn Reson Med. 2001;46(5):848-854.
2) Andia ME, Henningsson M, Hussain T, et al. Flow-independent 3D whole-heart vessel wall imaging using an interleaved T2-preparation acquisition. Magn Reson Med. 2013;69(1):150-157. doi:10.1002/mrm.24231.
3) Henningsson M, Koken P, Stehning C, Razavi R, Prieto C, Botnar RM. Whole-heart coronary MR angiography with 2D self-navigated image reconstruction. Magn Reson Med. 2012;67(2). doi:10.1002/mrm.23027.
4) Cruz G, Atkinson D, Henningsson M, Botnar RM, Prieto C. Highly efficient nonrigid motion-corrected 3D whole-heart coronary vessel wall imaging. Magn Reson Med. May 2016. doi:10.1002/mrm.26274.
5) Weigel M. Extended phase graphs: Dephasing, RF pulses, and echoes - pure and simple. J Magn Reson Imaging. 2015;41(2):266-295. doi:10.1002/jmri.24619.
Figures