4750
Imaging inflammation following MI using hyperpolarized pyruvate and 3D Spectral-Spatial EPI1Oxford Centre for Clinical Magnetic Resonance Research, University of Oxford, Oxford, United Kingdom, 2Department of Physics, University of Oxford, Oxford, United Kingdom, 3Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, United Kingdom
Synopsis
Current clinical imaging techniques offer only limited assessment of innate immune cell driven inflammation, which is an emerging therapeutic target in myocardial infarction. However, macrophages have a defined metabolic phenotype and are highly glycolytic when activated following injury. Here we show that hyperpolarized [1-$$$^{13}$$$C]pyruvate imaging specifically detects this phenotype, which is altered by pharmacological blockade that modulates monocyte/macrophage inflammatory function both in vitro and in vivo. We conclude that cardiac hyperpolarized [1-$$$^{13}$$$C]lactate several days post insult reflects immunology, and not necessarily ischaemia.
Purpose
Innate immune cells are now understood to be potential therapeutic targets in myocardial infarction (MI) and atherosclerosis, which together are leading causes of mortality worldwide.[1,2] Hyperpolarized MRI represents a clinically translatable method to assess cardiac metabolic behaviour directly, and in particular the "superstar" molecule [1-$$$^{13}$$$C]pyruvate is both well placed to probe glycolysis and has appropriate nuclear properties to render it readily imageable. MI is known to induce an intense local inflammatory response in the days and early weeks following the event, including a biphasic monocyte-macrophage response: following an influx of monocytes with a primarily inflammatory phenotype which peak in number at day 3, the myocardium subsequently recruits monocytes with broadly reparative functions, which are the dominant population by day 7. As activated monocytes/macrophages are known to be highly glycolytic (and thus would produce a high [1-$$$^{13}$$$C]lactate signal) we therefore propose to investigate the role of immunology following MI with hyperpolarized magnetic resonance.Methods
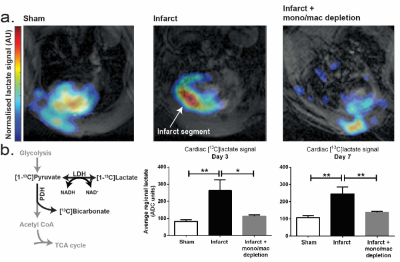
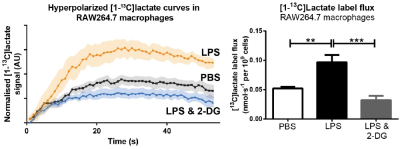
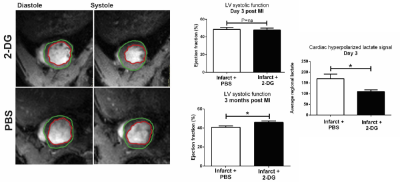
Hyperpolarized [1-$$$^{13}$$$C]pyruvate was administered to rats at day 3 or day 7 following experimental cryoinjury-induced MI or corresponding sham surgery, and a custom-designed 3D spectral-spatial echo-planar imaging sequence and reconstruction pipeline was used to measure regional myocardial metabolite signal intensity with a resolution of $$$2\times2\times4\,\,\text{mm}^{3}$$$ in a 7T Varian MRI system.[3,4] Briefly, this sequence acquires spatially resolved pyruvate, bicarbonate and lactate volumes with a 1s temporal resolution with an alternating phase encode direction that is used by the subsequent reconstruction to infer off-resonance and susceptibility artefacts that ordinarily make hyperpolarized imaging difficult. A further population of infarcted rats were subject to monocyte and macrophage depletion via clodronate liposomes and scanned as above. To see the effect of inhibiting "first wave" monocytes, 2-deoxyglucose (2DG) was administered to block glycolysis at hexokinase to rats for three days after either sham or MI. To test macrophage glycolytic flux directly, RAW264.7 macrophage-like cell suspensions were infused with hyperpolarized pyruvate on an 11.7T vertical-bore Bruker MR system with or without the activating agent lipopolysaccharide (LPS) and with or without 2DG. Simple pulse/acquire spectroscopy (TR=1s, sw=20 kHz, FA $$$\approx5^\circ$$$) was started concomitantly with the infusion of pyruvate directly into warmed cell cultures placed in a warmed 10 mm NMR tube.Results
As hypothesised, hyperpolarized [1-$$$^{13}$$$C]lactate signal increased in the untreated, infarcted groups compared to sham animals. Depletion of the monocyte/macrophage population using clodronate liposomes normalised the [1-$$$^{13}$$$C]lactate signal at both timepoints, despite equivalent volume and function measurements from cine MRI, implying equivalent infarct sizes. (Fig. 1). Flow cytometry revealed that monocytes/macrophages were present in large numbers, and expressed biphasic cell surface markers reflecting differential temporal expression (CD43$$$^\text{lo}$$$his48$$$^\text{hi}$$$ at day 3 and and CD43$$$^\text{hi}$$$his48$$$^\text{lo/int}$$$ at day 7). In vitro, RAW264.7 macrophages were found to be highly glycolytic, with lactate production apparently doubled after incubation with LPS, which was normalised by 2DG (Fig. 2). In vivo, treatment with 1g/kg/day 2DG suppressed cardiac [1-$$$^{13}$$$C]lactate. Hearts had equivalent ventricular volumes and systolic function at day 3, demonstrating equivalent injury size. The ventricles in both groups dilated by around 30% between day 3 and 3 months post MI, however the rate of increase of end-systolic volume was attenuated in 2DG treated rats, with a 5% absolute improvement in ejection fraction at 3 months (Fig. 3).Discussion, Conclusion and Future Work
Traditionally, cardiac immunomodulation following MI is seen as a risky intervention owing to cardiac rupture and impaired healing that can occur following pharmacological steroid blockage,[5,6] although targeted immunomodulation has recently been shown to bring therapeutic benefit.[7] Owing to their myriad functions and hypoxic niches, macrophages are highly glycolytic at rest, and possess a large intracellular lactate pool size that varies in accordance with key enzyme expression that changes rapidly following insult. As lactate is understood to be a paracrine "danger" signal in its own right,[8] assessment of the lactate pool size might provide a more fundamental assessment of innate immune cell immuno-metabolic function following MI. We have demonstrated that hyperpolarized MR is uniquely placed to exploit to this fact, which may enable the realtime assessment of myocardial following MI. Furthermore, we have shown that treatment with 2DG may produce favourable effects on systolic function following MI, which, as newer and more specific agents are in advanced stages of development, indicates space for future trials. In direct contrast to the ischaemic tissue reported on by [1-$$$^{13}$$$C]lactate in the acute context,[9] here we have conclusively demonstrated that the role of the immune system cannot be neglected in hyperpolarized studies. Future work will aim to monitor patient specific wound healing and response to medical therapy following MI.Acknowledgements
We would to acknowledge the financial support of a Novo Nordisk Foundation Postdoctoral Fellowship, an EPSRC Doctoral Prize Fellowship (EP/M508111/1), the [UK] National Institute for Health Research Oxford Biomedical Research Centre Programme, BHF Fellowships (FS/10/002/28078, FS/14/17/30634, RG/11/9/28921) and the British Heart Foundation Oxford Centre of Research Excellence award.References
[1] N. G. Frangogiannis, Nat. Rev. Cardiol. 2014, 11, 255–265.
[2] F. K. Swirski, M. Nahrendorf, Science (80-. ). 2013, 339, 161–166.
[3] J. J. Miller, A. Z. Lau, D. J. Tyler, Magn. Reson. Med. 2017, DOI 10.1002/MRM.26839.
[4] J. J. Miller, A. Z. Lau, I. Teh, J. E. Schneider, P. Kinchesh, S. Smart, V. Ball, N. R. Sibson, D. J. Tyler, Magn. Reson. Med. 2015, 75, 1515–1524.
[5] B. H. Bulkley, W. C. Roberts, A. Hazani, E. Al., Am. J. Med. 1974, 56, 244–250.
[6] R. Roberts, V. DeMello, B. E. Sobel, Circulation 1976, 53, I204–6.
[7] F. Leuschner, et al., Nat. Biotechnol. 2011, 29, 1005–1010.
[8] L. Yang, M. Xie, M. Yang, Y. Yu, S. Zhu, W. Hou, R. Kang, M. T. Lotze, T. R. Billiar, H. Wang, L. Cao, D. Tang, Nat. Commun. 2014, 5, 4436.
[9] D. R. Ball, R. Cruickshank, C. A. Carr, D. J. Stuckey, P. Lee, K. Clarke, D. J. Tyler, NMR Biomed. 2013, 26, 1441–1450.
Figures