4748
Respiratory motion-corrected simultaneous myocardial viability PET and coronary MR angiography: initial clinical validation1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Nuclear Medicine, TU Munich, Munich, Germany, 3MR Research Collaborations, Siemens Healthcare, Frimley, United Kingdom
Synopsis
Cardiac PET-MR imaging has shown promising results for the comprehensive assessment of coronary artery disease. Here we present an initial clinical validation of a recently demonstrated respiratory motion-corrected PET-MR framework in patients with chronic total occlusion. Simultaneous visualization of the coronary lumen by Coronary MR Angiography (CMRA) and myocardial viability by 18F-FDG PET was compared against X-ray angiography and LGE-MRI. We demonstrate that the proposed framework produces diagnostic images in both modalities in a short and time-efficient examination of ~12 minutes. Motion correction improved visible length and sharpness of the coronary arteries by CMRA and delineation of the myocardium and noise reduction by 18F-FDG PET, resulting in good agreement with X-ray angiography and LGE-MRI.
Introduction
Cardiac PET-MR has shown promising results for the comprehensive assessment of coronary artery disease1. However, most of the current developments for cardiac PET-MR focus on improving PET image quality by using motion information estimated from MR2. In these approaches, diagnostic MR images are typically acquired after the simultaneous PET-MR acquisition, leading to long acquisition times. A novel framework for the simultaneous acquisition of diagnostic cardiac PET-MR images, allowing the visualisation of myocardial integrity by 18F-FDG PET and coronary lumen integrity by CMRA in a single efficient examination has recently been proposed3. Here we present an initial clinical validation of this method in a cohort of patients with known cardiovascular disease.Methods
The proposed method consists in a prototype ECG-triggered free-breathing CMRA sequence simultaneously acquired with list-mode cardiac PET data. Respiratory motion is estimated from MR images (2D image navigators and 3D data itself), so that non-rigid motion corrected 3D CMRA can be performed efficiently (100% scan efficiency, thus no data rejection and shorter overall scan time), while simultaneously providing non-rigid motion fields for MR-based PET respiratory motion correction for both the emission data and attenuation maps.
Fourteen patients (age 66.1±9.5 years, 9 males) with chronic total occlusion (CTO) of at least one coronary artery were scanned on a Biograph mMR scanner (Siemens Healthcare). The clinical PET-MR routine included a 40 to 50 minutes insulin-clamped 18F-FDG list-mode PET acquisition for the assessment of myocardial viability, and a multi-slice 2D-PSIR LGE acquisition (1.4-2.2mm in-plane resolution, 8mm slice thickness). During the 10-15 minutes waiting time required for optimal contrast in LGE images, an acquisition with the proposed motion corrected PET-CMRA framework was performed with the following parameters: resolution = 1x1x2mm, FOV = 304x304x88-112mm, coronal orientation, TR/TE = 3.7/1.7ms, flip-angle = 15º, Fat Sat, T2Prep duration = 50ms and acquisition window = 89-119ms (depending on the length of the mid-diastolic rest period). All patients underwent interventional X-ray angiography the day after the PET-MR examination.
CMRA and PET data were reconstructed with the proposed motion correction scheme (MC) and without motion correction (NMC). The fraction of the list-mode PET data acquired simultaneously with the CMRA sequence was considered for reconstruction. Due to variations in the planning time, the overlap between the CMRA and list-mode PET acquisition varied between patients (8.8±1.2 min). PET image reconstruction was performed offline using reconstruct-transform-average4 motion correction in Siemens e7 Tools (OSEM, 3 iterations, 21 subsets, PSF modelling, 2.03x2.08x2.08mm voxel size, 127x344x344 matrix size).
Results
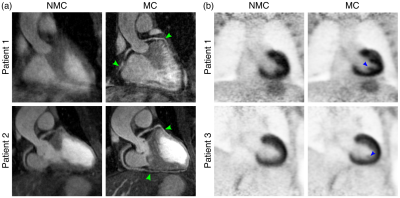
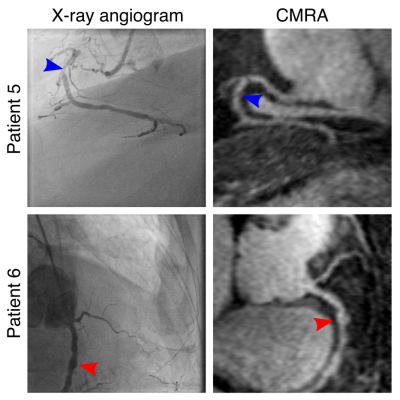
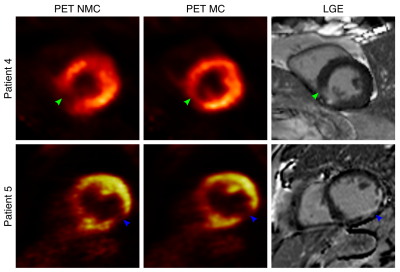
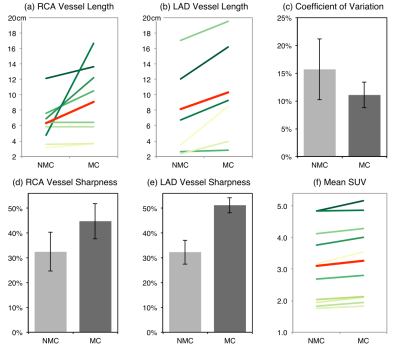
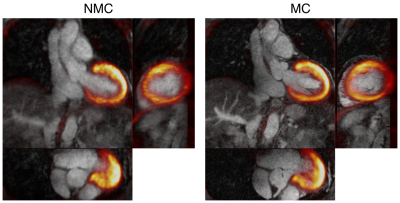
Scans were completed successfully in all subjects. The average acquisition time for the proposed PET-CMRA acquisition was 11.2±2.4 minutes. In two of the patients, there was no overlap between the PET and the CMRA acquisition, and one of the patients was not able to receive the Gadolinium-based contrast agent. These three patients were excluded from the following analysis. CMRA images were reformatted to simultaneously visualise the left anterior descending (LAD) and right coronary (RCA) arteries. Improvements in the LAD and RCA visualisation are observed after applying motion correction to the CMRA images (Fig1a). MC CMRA images show sufficient quality for depicting the proximal coronary anatomy, in agreement with X-ray angiogram findings (Fig2). The improvement is reflected in an increased visible length of both the RCA (+49,9% on average) and the LAD (+32,6% on average) (Fig4a-b); and in the vessel sharpness increasing by 12,3% and 18,9% on average for the RCA and LAD respectively (Fig4d-e). In the cardiac PET images, motion correction improves the visualisation of small structures (Fig1b) and improves the delineation of myocardial defects (Fig3). In particular, the degree of transmurality of viability defects is better depicted in the MC PET images and an improved agreement with the LGE was observed (Fig3). Mean SUV, in a spherical volume of interest in the myocardium, increased for all patients after motion correction, indicating an increased sharpness of the myocardium. The reduced coefficient of variation reflects the observed noise reduction in the MC PET images (Fig4c,f). Uncorrected (NMC) and motion-corrected (MC) fused PET-CMRA images are shown in Fig5.
Conclusion
We have presented a first clinical validation of a novel respiratory motion-corrected cardiac PET-MR framework for simultaneous visualisation of coronary anatomy and myocardial integrity in patients with coronary artery disease. Results show good agreement between the coronary anatomy depicted by CMRA and X-ray angiography. In addition, motion corrected 18F-FDG PET images were in good agreement with LGE-MRI and show more accurate depiction of both transmural and non-transmural viability defects.Acknowledgements
This work is funded by the King’s College London & Imperial College London EPSRC Centre for Doctoral Training in Medical Imaging (EP/L015226/1) and EPSRC Grant EP/N009258/1.References
(1) Rischpler C et al. Hybrid PET/MR imaging of the heart: potential, initial experiences, and future prospects. J Nucl Med 2013;54:402–415
(2) Kustner T et al. MR-based respiratory and cardiac motion correction for PET imaging, Med Imag Analysis 2017;42: 129-144
(3) Munoz C et al. Motion-corrected simultaneous cardiac positron emission tomography and coronary MR angiography with high acquisition efficiency. Magn Reson Med 2017. doi: 10.1002/mrm.26690
(4) Picard Y et al. Motion correction of PET images using multiple acquisition frames. IEEE Trans Med Imaging 1997;16:137–144
Figures