4745
Incorporating Motion-Sorting Technique into Keyhole and k-t GROWL Compound System for Rapid Golden-angle Liver DCE Imaging1Department of Biomedical Engineering, Zhejiang University, Hangzhou, China, 2Wuhan Institute of Physics and Mathematics (WIPM) of Chinese Academy of Sciences, Wuhan, China, 3Philips Healthcare, Shanghai, China, 4School of Biomedical Engineering, Guangdong Provincial Key Laborary of Medical Image Processing, Southern Medical University, Guangzhou, China, 5South China University of Technology, Guangzhou, China, 6School of Information Technology and Electrical Engineering, The University of Queensland, Brisbane, Australia
Synopsis
Liver DCE imaging plays an increasingly important role in the diagnosis of liver diseases, including hepatic cirrhosis, hepatocellular carcinoma, etc. Motion is an inevitable problem in liver imaging, which often leads to motion artifacts and blurring on image details. We propose to incorporate motion-sorting technique into parallel imaging GROWL and Keyhole compound system for golden-angle radial dynamic contrast-enhanced MRI. The experimental results demonstrated that the proposed scheme can generate better image quality than non-motion-sorting techniques. Compared to the tested motion-sorting techniques, similar image quality can be offered with greatly reduced computational cost.
Target Audience
Radiologist and scientists who are
interested in rapid liver perfusion and real-time imaging.
Purpose
In liver MRI, organ movements caused mainly by respiration often results in motion artifacts and blurring on edge or detail1. Perfusion imaging like dynamic contrast-enhanced (DCE) MRI has a crucial role to play in the study of liver diseases. Currently, some motion-robustness liver DCE-MRI techniques have attracted much attention of scholars to fulfill the need for perfusion imaging2–4; however, these approaches involve iterative SENSE-based solution procedure that are computationally expensive and sensitive to parameter5, thus impeding its clinical application. The aim of this study is to propose a non-iterative rapid DCE imaging approach which combines motion-sorting technique4, keyhole6 and parallel imaging GROWL7 without regularization parameter selection. Compared to non-motion-sorting techniques, the proposed scheme provides a solution with less motion artifacts as well as better image quality. Compared to motion-sorting techniques, the proposed scheme generates similar reconstruction. More importantly, the proposed scheme is able to obtain satisfactory results with greatly reduced computational cost (10 times lower than similar techniques), thus practical in clinical applications.Methods
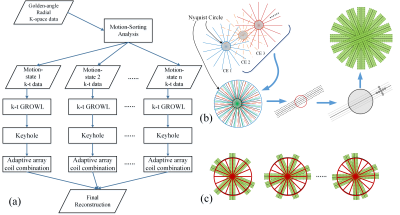
We propose to incorporate a motion-sorting scheme into keyhole and parallel imaging GROWL system for rapid liver DCE imaging. In the proposed scheme, a motion-sorting technique is performed first in free-breathing golden-angle radial sampling4, and then GROWL is modified as k-t GROWL, which is to perform calibration kernel within a predefined full neighborhood over all coils and time frames from all motion states (Fig. 1b). The Keyhole algorithm is also modified to combine with GROWL in our scheme (Fig. 1c). The complete framework displayed in Fig.1a is analyzed as follows:
(S1) The motion-state signals are extracted from k0 points of each spoke, and then the radial k-space data is sorted into a specific number of motion states.
(S2) For each motion state in previous step, a GROWL operator is performed for each radial spoke. In the calibration procedure of this GROWL, original non-sorted k-space data is used to further improve the accuracy.
(S3) The k-space data of motion state 1 is sorted into several contrast-enhancement phases. A keyhole operator is performed in each state, and the radius of the hole can be approximately calculated by:
R=Ns/(π*FOV)
Where Ns is the number of acquired spokes of each motion-state.
(S4) (S3) is repeated for every motion state; afterwards a rapid gridding operator8 is used to restore the to-be-reconstructed image of each contrast-enhancement phases.
The liver DCE imaging experiment was performed on a 3.0 T Verio MR scanner (Siemens AG Medical Solutions, Erlangen, Germany) using the standard 20-element body/spine coil array. A radial stack-of-stars 3D FLASH pulse sequence with normal-breathing golden-angle scheme was employed for this acquisition. The relevant parameters included: FOV 360 × 360 × 240 mm3, TR/TE ≈ 3.52/1.41 ms, number of slices 38, number of readout points in each spoke 512, oversampling ratio 2, number of spokes 1100, and slice thickness 3 mm. Frequency-selective fat suppression was used for both acquisitions.
In this study, the entire dataset was sorted into 4 motion states, 11 DCE phases, 25 radial spokes per frame. All image reconstructions were implemented in Matlab (R2015b; the Mathworks, Natick, MA, USA), for off-line reconstruction on an Inspur Yingxin Server (NP 3588) with 8-Core 2.67GHz Intel i7 CPU and 12GB of Memory. As for the efficiency evaluation, the CPU time of each method was recorded.
Results & Discussion
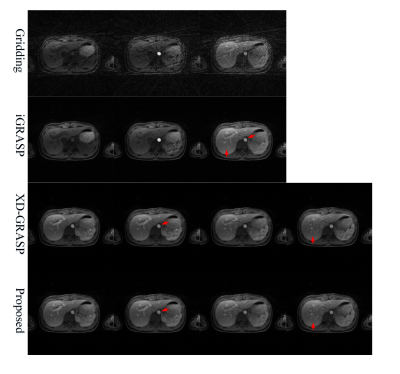
As displayed in Fig.2, the proposed scheme generates better image quality than non-motion-sorting scheme iGRASP. We can see better edge sharpness and more image details, as the red arrows pointed in the figure. When comparing the proposed scheme with motion-sorting scheme like XD-GRASP, the reconstructed image quality of the proposed technique is comparable to the latter. Moreover, as shown in Table I, the proposed scheme is 10 times faster than either the iterative iGRASP or XD-GRASP schemes. Theoretically, the predefined coil sensitivity maps (CSM) will introduce errors due to motion effect. Here, the adaptive array coil combination9 is used motion-state by motion-state to improve the accuracy of reconstruction instead of the fixed CSM in previous schemes.Conclusion
We have proposed a hybrid image reconstruction algorithm that involves a non-iterative motion-sorting scheme, which is effective for liver DCE imaging. The experimental results indicated that, compared to non-motion-sorting techniques, it has the advantage of offering high image quality, with less motion artifacts, clearer edge, and better anatomical details. Compared with motion-sorting techniques, it offers similar image quality but being much more efficient. This can be of great potential value for real-time imaging in clinical application.Acknowledgements
We thank the Center for Advanced Imaging Innovation and Research (CAI²R) in NYU for their generously sharing the liver DCE dataset.References
[1] Lin W, Guo J, Rosen MA., et al. Respiratory Motion-Compensated Radial Dynamic Contrast-Enhanced (DCE)-MRI of Chest and Abdominal Lesions. Magn Reson Med. 2008; 60(5): 1135–1146. [2] Feng L, Grimm R, Block KT, et al. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. 2014; 72(3): 707–717. [3] Otazo R, Candès E, Sodickson DK. Low-rank plus sparse matrix decomposition for accelerated dynamic MRI with separation of background and dynamic components. Magn Reson Med. 2015; 73(3): 1125–1136. [4] Feng L, Axel L, Chandarana H, et al. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med. 2016; 75(2): 775–788. [5] Pruessmann KP, Weiger M, Börnert P, et al. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn Reson Med. 2001; 46(4): 638–651. [6] van Vaals JJ, Brummer ME, Dixon WT, et al. "Keyhole" method for accelerating imaging of contrast agent uptake. J Magn Reson Imaging. 1993; 3(4): 671–675. [7] Lin W, Huang F, Li Y, et al. GRAPPA operator for wider radial bands (GROWL) with optimally regularized self-calibration. Magn Reson Med. 2010; 64(3): 757–766. [8] Beatty PJ, Nishimura DG, Pauly JM. Rapid gridding reconstruction with a minimal oversampling ratio. IEEE Trans. Med. Imaging. 2005; 24(6): 799–808. [9] Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn Reson Med. 2000; 43(5): 682–690.Figures