4741
Relevance of susceptibility weighted imaging with phase (SWIp) in Cholelithiasis1Philips Innovation Campus, Philips India Limited, Bengaluru, India, 2Fortis Memorial Research Institute, Gurugram, India, 3Philips India Limited, Gurugram, India
Synopsis
Cholelithiasis, which is presence of stones in the biliary anatomy, is a common clinical condition with an incidence of up to 15% worldwide. Magnetic resonance cholangiopancreatography (MRCP) is a standard MR technique used for diagnosing this condition. However, MRCP’s sensitivity is low when the stones are smaller than 5mm. These stones often contain trace amounts of minerals that are dia/paramagnetic. SWI is highly sensitive to susceptibility differences and hence we hypothesized that it may play a role in clinical evaluation of presence of gallstones. In this work we evaluated this hypothesis by imaging patients with Cholelithiasis using a modified SWI sequence.
Introduction
Cholelithiasis, i.e., presence of stones in the gallbladder, is a common condition which has an incidence of 15% in USA, 22% in Europe and 4-15% in Asia.1–3 Accurate diagnosis of the presence and location of gallstones is the key to appropriate treatment planning. Trans-abdominal Ultrasound (US) is the first line of clinical imaging. However, US is highly operator dependent and in case of stones in the common bile duct, detection sensitivity of US drops to less than 50%.4 Magnetic resonance cholangiopancreatography (MRCP) is another non-ionizing, non-invasive imaging method that was introduced in 19915 and is routinely used today for MRI based assessment of biliary conditions.6 However, in detecting stones smaller than 5 mm, MRCP’s sensitivity is only 62-64%.7 Furthermore, MRCP is often susceptible to patient motion and could also be influenced by B1 inhomogeneities. While the constituents of the calculi (stones) varies, about 28% of them contain calcium8 and 71% contain cholesterol and even cholesterol stones contain trace amounts of calcium salt.9 Calcium has distinct magnetic susceptibility (diamagnetic) relative to the surrounding tissue and cholesterol, due to its lipid content, has both chemical shift and susceptibility10 (paramagnetic). In view of this, we hypothesized that phase from MR susceptibility weighted imaging (SWI) technique could serve as a useful adjunct in clinical imaging of calculi in biliary anatomy.Methods
All imaging was performed on a Philips 3.0T Ingenia using dStream torso coil along with the spine coil. The conventional 3D multi-echo SWI sequence (SWIp sequence) was modified to: TR 12msec, TE 7msec, flip angle 8o, voxel size 1x 1x 6 mm3. The sequence was applied in 197 consecutive patients receiving a diagnostic MRI scan for either a clinically indicated MRCP or a routine whole-body health screening. A total of 18-28 slices were acquired within 30-40seconds under breath-hold conditions. The magnitude SWIp sequence data was used for diagnostic purposes as a T2* weighted imaging technique. Imaging data from subjects with surgically confirmed gall bladder stones was obtained and the filtered phase11,12 from SWIp sequence was evaluated for visualization of the calculi by an experienced radiologist. Furthermore, in cases where stones were visualized, the nature of the phase, i.e., diamagnetic vs. paramagnetic or mixed phase, was characterized along with the mean phase value from the central slice.Results
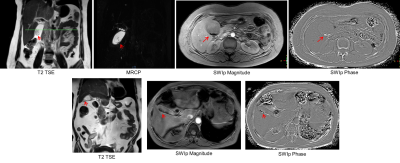
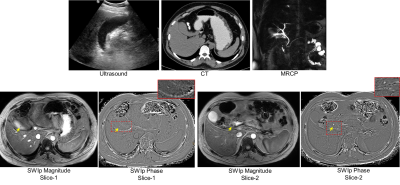
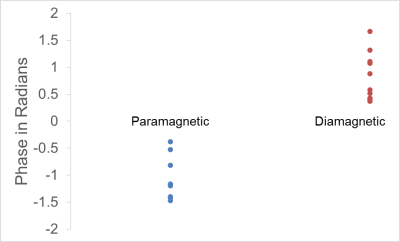
Thirty one subjects had radiologically identified calculi which were subsequently confirmed after cholecystectomy. SWI data from two subjects was excluded due to severe motion and lack of sufficient coverage. Thus 29 subjects were evaluated. In 28 of these, the calculi were clearly visualized in SWI with phase imaging. Figure-1 shows the collage comparing visualization of calculi in conventional protocol and SWI phase images in two subjects. In one of the subjects, gall stones were visualized only in the SWI data due to their small size. Interestingly, CT and Ultrasound investigations also failed to identify the stones in this patient with clinical features of cholecystitis (figure-2). Phase signature of the stones was diamagnetic in 13 cases (0.78+/-0.43 radians), paramagnetic in 8 (-1.04+/-0.42 radians) and mixed in 7 of the cases. Figure-3 shows the spread of the phase values from the stones in-vivo.Discussion
The results confirm that the modified 3D-SWI sequence is sensitive in visualizing gallstones. There was considerable variability in the phase signature of the stones (dia vs. paramagnetic) as well as in its value. Sensitivity of SWI phase is significantly modulated by TE and composition, structure and size of the stone. A long TE and thin slices (relative to stone-size) typically helps with improved phase sensitivity. However, in this work, a short TE and a thick slice along with a short TR was used which was necessary to bring down the imaging time. Use of motion resilient trajectories like radial, which allow imaging under free-breathing condition, could enable use of longer TE’s and thinner slices. Distinct phase signature is not expected for all stones due to the variability in their composition. Nevertheless, in the cohort studied, calculi were visualized in SWI in 28/29 cases (97%). This may be due to high sensitivity of SWI phase to the magnetic susceptibility of biliary calculi. Further studies with larger sample size would be needed to investigate the sensitivity of SWI in cholelithiasis.Conclusion
SWI helps in the visualization of biliary calculi and could serve as an important adjunct to conventional diagnostic imaging protocol for biliary conditions to enhance diagnostic confidence. This is the first demonstration of the potential role of SWI in imaging biliary stones.Acknowledgements
No acknowledgement found.References
1. Everhart, J. E., Khare, M., Hill, M. & Maurer, K. R. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology 117, 632–639 (1999).
2. Aerts, R. & Penninckx, F. The burden of gallstone disease in Europe. Aliment. Pharmacol. Ther. 18 Suppl 3, 49–53 (2003).
3. Zhang W, Jiang Z, H. T. & R, L. Epidemiology and risk factors of cholelithiasis. J Surg Concepts Pr. 16, 408–412 (2011).
4. Einstein, D. M., Lapin, S. A., Ralls, R. W. & Halls, J. M. The insensitivity of sonography in the detection of choledocholithiasis. Am. J. Roentgenol. 142, 725–728 (1984).
5. Wallner, B. K., Schumacher, K. A., Weidenmaier, W. & Friedrich, J. M. Dilated biliary tract: evaluation with MR cholangiography with a T2-weighted contrast-enhanced fast sequence. Radiology 181, 805–8 (1991).
6. Costi, R., Gnocchi, A., Di Mario, F. & Sarli, L. Diagnosis and management of choledocholithiasis in the golden age of imaging, endoscopy and laparoscopy. World J. Gastroenterol. 20, 13382–13401 (2014). 7. Boraschi, P. et al. Choledocolithiasis: Diagnostic accuracy of MR cholangiopancreatography. Three-year experience. Magn. Reson. Imaging 17, 1245–1253 (1999).
8. Qiao, T. et al. The Systematic Classification of Gallbladder Stones. PLoS One 8, (2013).
9. Kaufman, H. S., Magnuson, T. H., Pitt, H. A., Frasca, P. & Lillemoe, K. D. The distribution of calcium salt precipitates in the core, periphery and shell of cholesterol, black pigment and brown pigment gallstones. Hepatology 19, 1124–1132 (1994).
10. Szczepaniak, L. S., Dobbins, R. L., Stein, D. T. & Denis McGarry, J. Bulk magnetic susceptibility effects on the assessment of intra- and extramyocellular lipids in vivo. Magn. Reson. Med. 47, 607–610 (2002).
11. Yoneda, T. & Hiai, Y. Phase difference enhanced imaging method (padre), functional image creating method, phase difference enhanced imaging program, phase difference enhanced imaging apparatus, functional image creating apparatus, and magnetic resonance imaging (mri) apparatus. (2017).
12. Chen, Z., Gilbert, G. & Fuderer, M. Improved contrast in multi-echo susceptibility-weigthed imaging by using non-linear echo combination. Int. Soc. Magn. Reson. Med. 23, 1730 (2015).
Figures