4730
Preliminary Study on the Feasibility of Simultaneously Measuring Hepatic Stiffness and Separate Water/Fat Signal Using Dual-Echo, Dixon, Spoiled-Gradient-Echo, Magnetic Resonance ElastographyYuan Le1, Joshua Trzasko2, Kevin Glaser2, Yuxiang Zhou3, William Pavlicek1, Joseph M. Hoxworth3, Bradley D. Bolster Jr.4, Joel P. Felmlee2, Richard L. Ehman2, and Jun Chen2
1Radiology, Mayo Clinic Arizona, Scottsdale, AZ, United States, 2Radiology, Mayo Clinic, Rochester, MN, United States, 3Radiology, Mayo Clinic Arizona, Phoenix, AZ, United States, 4Siemens Medical Solutions USA, Inc., Salt Lake City, UT, United States
Synopsis
A novel dual-echo, Dixon MR Elastography technique was developed to simultaneously measure the liver stiffness and separate fat/water signal. Phantom tests showed that the fat signal fraction and the stiffness measured with this technique were consistent with the values measured with separate MR spectroscopy and standard MR Elastography acquisitions. Promising results were also obtained in a healthy volunteer.
Introduction
MR Elastography (MRE) sequences are part of a class of phase-difference sequences that use specialized gradient waveforms to encode harmonic motion into the phase of MR images1, 2. The purpose of this study was to demonstrate the feasibility of using a novel, dual-echo, Dixon MRE technique to simultaneously measure hepatic stiffness and separate water and fat signal3, 4. This new technique circumvents the need for per-axis, concomitant-field corrections while reducing scan time, which is clinically important for applications such as liver MRI 5, 6.Materials and Methods
Fig. 1 shows the dual-echo, GRE-MRE pulse sequence, with motion-encoding gradients in the slice-selection direction. Images were reconstructed in two stages. First, for each echo, MRE displacement maps (phase images) were estimated, from which tissue stiffness maps were derived. Second, motion-induced phase was demodulated from all images in the series, and separate water and fat images were derived. One MRE PVC QC phantom, one water-fat-bovine-gel (WFBG) phantom and a volunteer were scanned on clinical 3T scanners (Skyra, Siemens Healthineers, Erlangen, Germany). The WFBG phantom (as shown in Fig. 2) was made with 1500 mL of water, 750 mL of vegetable oil, 22 envelopes (~141g) of Knox Gelatine (bovine gel) and 70 g of emulsifying wax NF (MilliardTM, Milliard Brands, Lakewood, NJ)7, 8. The TR/TEs for the PVC phantom and the volunteer were TR/TE1/TE2 = 37.5/21/25 ms (~OP/IP). For the WFBG phantom they were TR/TE1/TE2 = 604/30/33ms (~IP/OP). The FOV was 240, 300, and 300 mm for the PVC phantom, the WFBG phantom, and the volunteer, respectively. The acquisition matrix was 128x64 and the frequency of the MRE motion was 60 Hz. The fat signal fraction of the WFBG phantom was measured using T2-corrected, single-voxel, multi-echo, 1H MRS (HISTO, TR = 1500 ms, TE=12, 24, 36, 48 and 72 ms) and single-voxel spectroscopy (SVS, TR = 600 ms, TE = 30 ms). The stiffness of both phantoms was measured using a standard GRE MRE sequence (TR = 50 ms, TE = 20.75 ms). Because the two echos were not acquired exactly at the IP and OP TE times, the phase angle between the water and fat signals was taken into consideration.Results
Phase-based displacement images were obtained with visible shear waves from both readouts in phantoms and the volunteer. The PVC phantom stiffness was measured to be 6.1±0.2 kPa and 6.1±0.2 kPa using the first and second echoes, respectively. The stiffness of the same phantom measured with standard GRE MRE was 5.9±0.1 kPa (Fig. 3). For the WFBG phantom, the fat signal fraction was measured to be 35% using HISTO (36-ms TE), 31% with spectroscopy, and 39% with dual-echo MRE (Fig. 4). The wave images for this phantom from both dual-echo MRE and standard GRE MRE showed similar wavelengths that were too long for an accurate stiffness measurement. In the volunteer study, the liver stiffness from each of the two echoes was 2.6±0.3 kPa and 2.3±0.3 kPa (Fig. 5). The IP/OP images also exhibit classic chemical shift characteristics at water-fat boundaries.Discussions
The stiffness of the PVC phantom measured using dual-echo MRE was very close to the stiffness measured by standard MRE. For the WFBG phantom, we could not accurately measure the stiffness of this very stiff phantom. However, the similarities in the wave information between the dual-echo MRE sequence and standard MRE acquisitions demonstrate the potential of the dual-echo MRE technique. The fat signal fraction measured with dual-echo MRE is slightly higher than the result from HISTO as well as SVS, which may be caused by residual T2* or B0 inhomogeneity effects in the dual-echo method. In future studies we will develop phantoms with realistic liver stiffness values and fat signal fraction to further optimize dual-echo Dixon MRE for liver stiffness and fat quantification, incorporate complete Dixon processing, and develop multi-echo (>2) MRE to measure additional liver properties like R2*. There may be additional challenges with multipoint Dixon MRE as the SNR will be lower with the longer TEs required for MRE, and this will have to be taken into account during the development of the technique.Conclusions
This study demonstrates the feasibility of a single-scan, dual-echo, Dixon MRE technique for liver stiffness and fat quantification, providing stiffness and fat concentration estimates that are consistent with results obtained using separate MRE and fat quantification scans.Acknowledgements
No acknowledgement found.References
1. Litwiller, D.V., et al., Magnetic Resonance Elastography. Curr Med Imaging Rev, 2012. (8)(1): p. 46-55. 2. Muthupillai, R., et al., Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science, 1995. (269)(5232): p. 1854-7. 3. Trzasko, J.D., et al., Simultaneous MR Elastography and Fat+Water Imaging. in Proc. ISMRM. 2015. Toronto, Canada. 1061 4. Numano, T., et al., Integration of MR Elastogeraphy and Fat/Water Separation Imaging. in Proc. ISMRM. 2017. Honolulu, Hawaii. 1381 5. Venkatesh, S.K., et al., Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging, 2013. (37)(3): p. 544-55. 6. Chen, J., et al., MR Elastography of Liver Disease: State of the Art. Appl Radiol, 2013. (42)(4): p. 5-12. 7. Hines, C.D., et al., T1 independent, T2* corrected MRI with accurate spectral modeling for quantification of fat: validation in a fat-water-SPIO phantom. J Magn Reson Imaging, 2009. (30)(5): p. 1215-22. 8. Le, Y., et al., Development and evaluation of TWIST Dixon for dynamic contrast-enhanced (DCE) MRI with improved acquisition efficiency and fat suppression. J Magn Reson Imaging, 2012. (36)(2): p. 483-91.Figures
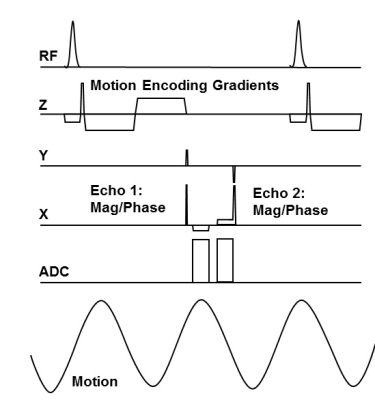
Figure 1. Timeline of the pulse sequence.

Water-fat-bovine-gel phantom.
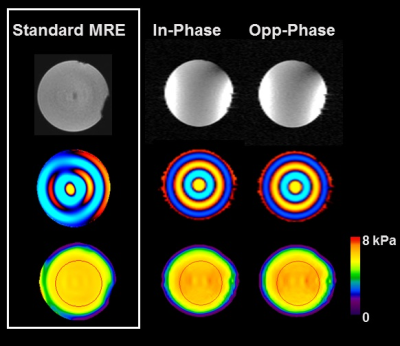
Dual-echo MRE and standard MRE images of the PVC
phantom.
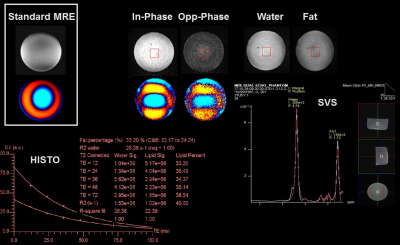
MRE Images and spectroscopy results from the
water-fat-bovine-gel phantom. The water and fat images were calculated from the
IP/OP images. Fat signal fraction was measured with HISTO and SVS. Wave images from
both standard and dual-echo MRE showed that the wavelength was too long for
accurate stiffness measurement in this phantom.
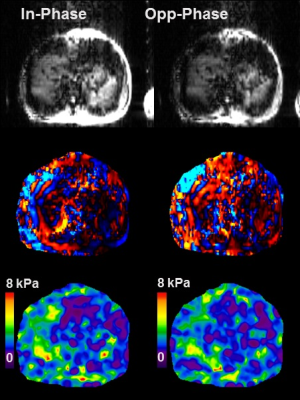
IP and OP images from the volunteer study and
the measured stiffness.