4726
Assessment of liver dysfunction with 1H-MRS and relaxometry in a murine model of cerebral malaria1Centre de Résonance Magnétique Biologique et Médicale UMR CNRS 7339, CNRS-Aix Marseille Université, Marseille, France
Synopsis
Cerebral malaria (CM) is the most lethal complication of Plasmodium infection and may be associated with multiple organ failure. We have investigated liver microstructure and metabolism in a widely-used murine model of CM induced with Plasmodium Berghei ANKA using in vivo anatomical MRI, relaxometry and localized proton spectroscopy at 11.75T. Our results show an increase in liver T2 value with CM progression. This increase is apparently linked to a reduction in tissue water content and to lipid remodeling. These alterations could be related to the clinical degradation occurring at the severe stage of the disease as well as to a direct effect of the parasite on the liver.
Introduction
Cerebral malaria (CM), the most lethal complication of Plasmodium infection, leads to death in 15-20 % of the cases, mostly children under five in sub-saharan Africa. CM is often difficult to recognize in Plasmodium-infected populations because of concomitant diseases. Moreover, CM is frequently associated with multiple organ failure in adults, which may worsen neurological signs. A widely-used experimental model of pediatric cerebral malaria (ECM) is obtained by infecting susceptible mice with Plasmodium Berghei ANKA (PbA)1. Recently, it was suggested that other organs than brain could be affected by PbA in ECMl2. A better knowledge of this model is required to improve our understanding of ECM physiopathology in a context of multiple organ failure. In this pilot study, we investigated liver structure and metabolism during ECM by using an approach combining anatomical MRI, relaxometry and proton spectroscopy.Methods
C57BL/6J mice were inoculated with parasitized red blood cells and monitored daily for the clinical/neurological signs of ECM1. Healthy controls and infected animals were imaged from d5 to d8 after PbA injection. The MR protocol was performed under isoflurane anesthesia at 11.75 T (vertical Bruker AVANCE 500 WB MR system). Transverse MR images (FOV 24×24 mm2, slice thickness 0.5 mm) were acquired using prospective respiratory gating (PC-SAM, Small Animal Instruments Inc). Anatomical imaging of the liver (matrix 240x240, in-plane resolution 100µm) was performed using a 2D spin-echo sequence (repetition time TR≥448 ms; echo time TE = 14 ms) with 20 contiguous slices. T2 maps (TR ≥ 9 s; 12 equally spaced echoes at TE = 7.5 to 120 ms; matrix 64×64, 2 accumulations) were acquired in a single slice. T2-maps were computed assuming mono-exponential decay3 using ImageJ. One large ROI was delineated, avoiding dorso-ventral phase-encoded flow artifacts on a slice devoid of respiratory motion artifact, and T2-histograms were analyzed. A ROI in the spinal cord was used as internal reference. A Kruskal-Wallis test followed by Dunn’s multiple comparison was applied on the average T2-values of the ROIs at each time point. 1H-MRS of the liver was performed at d7 only using the PRESS sequence (TE= 16.51ms, TR= 5000ms, voxel size= 27mm3) after shimming with FASTMAP. Both water-saturated (NS=32) and non-saturated spectra (NS=8) were acquired (saturation with VAPOR). Spectra were processed under CSIAPO using AMARES4. Several parameters related to lipid composition were derived from lipid resonances5 including the saturated lipid component (SL), the total of unsaturated lipids (ULtot), the fraction of unsaturated lipids (fUL), the fraction of saturated lipids (fSL), the fraction of polyunsaturated lipids (fPUL) and the fraction of monounsaturated lipids (fMUL). Mann-Whitney test was used to compare control and infected animals (significance set to P<0.05).Results
All infected mice developed ECM within 8 days following PbA injection and showed the usual clinical and neurological signs of the disease1. ECM was associated with body weight loss (ca 11% at d7 after PbA injection). Anatomical MRI did not reveal any overt hepatic lesion, but a reduction in subcutaneous fat was visible at the end of the follow-up concomitant with body weight loss. Metabolic results (control mice: n=4, ECM mice: n=4) showed a significant increase in fPUL (Figure 1) in ECM mice as well as a trend for a decrease in water signal intensity at d7 (Figure 2). Liver T2 values increased significantly with disease severity up to d6 or d7 and decreased thereafter (Figure 3), while T2-values in spinal cord did not change by more than 8%. On average, liver T2 was increased by 31% at d6 and by 22% at d7, compared to controls (Figure 4).Discussion/Conclusion
The T2 increase might be due to changes in interstitial water, protein content, lipid composition and/or lipid mass. Anomalies in water content and lipid composition were detected by 1H-MRS at the latest stage of ECM. The increase in fPUL reflects an increase in lipids with short T2 5, however still superior to the highest T2 value measured with relaxometry in this study (16 ms). Changes in T2 values measured by relaxometry might be partly explained by loss of free water as suggested by the spectroscopic results. A longitudinal 1H-MRS study on a larger cohort is required to better understand the origin of T2 variation and the extent of hepatic lipid remodeling in ECM. Such study could help determine whether the alteration of these parameters is a side-effect of acute clinical degradation (dehydration and body weight loss at the late stage of ECM), or a direct consequence of liver dysfunction caused by PbA.Acknowledgements
France Life Imaging (FLI).References
1-Penet MF, Viola A, Confort-Gouny S, et al. Imaging experimental cerebral malaria in vivo: significant role of ischemic brain edema. J Neurosci. 2005;25(32):7352-7358.
2-Ghosh S, Sengupta A, Sharma S, et al. Metabolic fingerprints of serum, brain, and liver are distinct for mice with cerebral and noncerebral malaria: a (1)H NMR spectroscopy-based metabonomic study. J Proteome Res.2012, 11: 4992-5004.
3-Masi B, Perles-Barbacaru TA, Laprie C et al. In Vivo MRI Assessment of Hepatic and Splenic Disease in a Murine Model of Schistosomiasis. PLoS Negl Trop Dis. 2015;9(9):e0004036.
4-Le Fur Y, Nicoli F, Guye M, et al. Grid-free interactive and automated data processing for MR chemical shift imaging data. MAGMA. 2010; 23(1):23–30.
5-Ye Q, Danzer CF, Fuchs A, Vats D, Wolfrum C, et al. Longitudinal evaluation of hepatic lipid deposition and composition in ob/ob and ob/+ control mice. NMR Biomed. 2013; 26: 1079-1088.
Figures
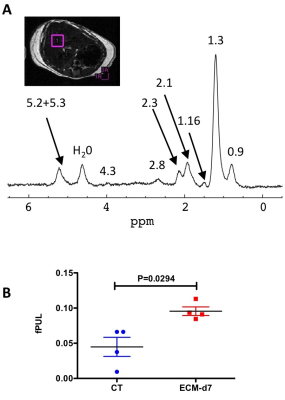
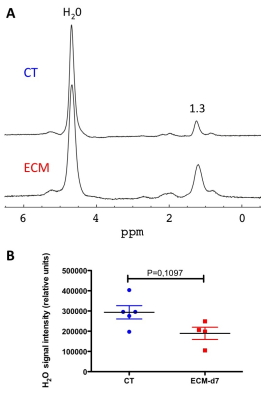
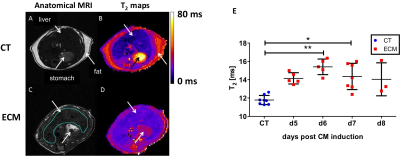
Fig.3:
Quantitative T2 mapping of liver. A and C: anatomical MRI. B and D: T2 maps. E: comparison of T2 values between CT and ECM mice.