4724
Fully Automated Liver Fat Assessment using Multi-Atlas Segmentation1Department of Radiology, Uppsala University, Uppsala, Sweden, 2Antaros Medical, BioVenture Hub, Mölndal, Sweden, 3Department of Medical Sciences, Uppsala University, Uppsala, Sweden
Synopsis
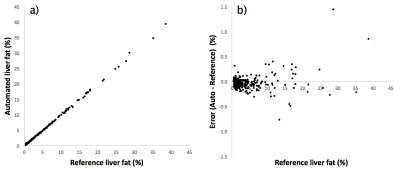
Non-alcoholic fatty liver disease (NAFLD) has become the most common liver disease with an estimated global prevalence of 25%. Its link to metabolic, cardiovascular, and more severe forms of liver disease presents a major challenge for future healthcare. MRI allows accurate quantification of liver fat concentration. Since manual delineation of the liver is time-consuming, measurements are typically performed in small subjectively selected regions of interest which limits accuracy and precision. This work presents an automated method for liver fat assessment using multi-atlas segmentation. Evaluation with measurements using manual liver segmentation (n=306) demonstrates excellent agreement (R=1.000, difference -0.03%, p=0.001).
Introduction
Non-alcoholic fatty liver disease (NAFLD) has become the most common liver disease with an estimated global prevalence of 25%.1 Its link to metabolic, cardiovascular, and to more severe forms of liver disease presents a major challenge for future healthcare. Despite bias from sampling errors, biopsy is considered gold standard for liver fat quantification. Biopsy also allows assessment of inflammation and fibrosis. Computed tomography, ultrasound and MR spectroscopy are alternative techniques.2 Water-fat MRI techniques that separate signals from water and fat using chemical shift allow accurate quantification of liver fat.3,4 The measurements from collected image data are however typically performed in small manually selected regions of interest. This limits measurement accuracy and precision.
Purpose
To develop and evaluate an automated method for accurate and precise liver fat quantification using large volume of interest liver segmentation from water-fat MRI using a multi-atlas approach.Methods
Water-fat MRI was successfully collected in the POEM-study from 306 subjects (156 males, BMI 26.4±4.3 kg/m2) in supine position with arms above the head using the body coil in a 1.5T MR system (Achieva, Philips Healthcare, The Netherlands). A 3D six-gradient echo sequence, with unipolar echoes, was collected with scan parameters: TR 8.7ms, TE 0.92ms, delta TE 1.32ms, flip 5 deg, FOV 384x288x150mm in sagittal x coronal x axial directions, acquired and reconstructed voxel size 3x3x10mm. Water-fat reconstruction was performed using a previously described software5 omitting the first echo time to reduce effects of eddy currents.6,7
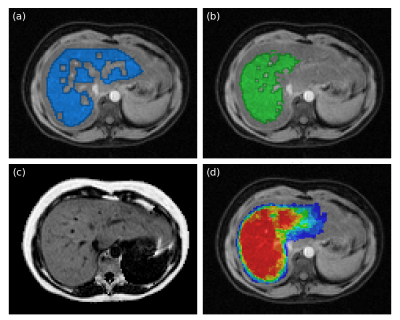
Reference segmentation was performed by an experienced operator delineating the outer contour of the liver using the water image. Central parts of the liver, where there is adipose tissue, larger blood vessels and bile ducts were included in the delineation but subsequently excluded using post processing for objective delineation of liver tissue. The post processing filtered outlier intensities in the water image by limiting the range to the mean±2 standard deviations. Erosion using a 3x3x3-kernel was applied to reduce partial volume effects from neighboring non-liver tissues.
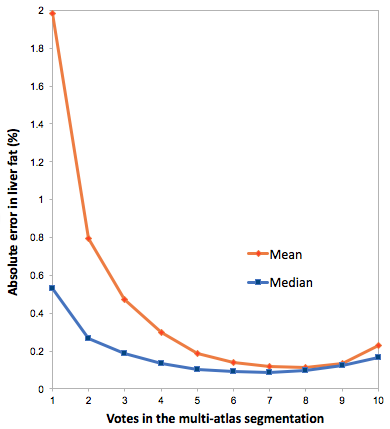
The automated segmentation was achieved using a multi-atlas approach. The atlas consisted of manual segmentations of ten livers from a previous study on the same MR system. The outer liver border was avoided to reduce partial volume effects. The water images from the ten livers in the atlas were registered to each water image from the POEM study. The individual segmentations were deformed using the corresponding transformation and label voting was used to determine the final segmentation. The effect of different number of votes was examined and seven was used for final readouts.
The registration was performed using the Elastix (v4.7) toolbox,8 using an affine pre-registration (3 resolutions) followed by a deformable registration optimizing a transformation parameterized by B-splines (grid spacing 24x24x24 mm). An adaptive stochastic gradient descent9 maximizing mutual information was used for both affine (500, 500, 1000 iterations) and deformable registrations (1000 iterations).
Liver fat estimates using both mean and median values from the segmented regions were evaluated. The measurements were compared using Pearson correlations, absolute differences and t-tests.
Results
Image examples including reference and automated segmentations are shown in Fig 1. Statistics on the liver fat measurements are shown in Tab 1. Scatter plots of measurements and errors are shown in Fig 2. The effects of different number of votes for the multi-atlas registrations is shown in Fig 3. The average total time for the multi-atlas registration was 4 minutes and 34 seconds and for the manual reference segmentation approximately 6 minutes.
Discussion
A method for fully automated quantification of liver fat using multi-atlas segmentation of large liver volumes of interest from water-fat MRI has been described. The automated measurements show excellent correlation to manually created reference segmentations in a large dataset. The measurements are however slightly but significantly underestimated. As shown in Fig 3 the use of the median value makes the assessment robust despite the reduced segmentation quality at lower multi-atlas votes. Previously also alternative methods have been presented for liver segmentation.10–12 Future work includes comparison to some of these alternative methods and evaluation of performance on multi-site and multi-vendor datasets and effect of use of different number of segmentations in the atlas database.
Acknowledgements
Swedish Research Council (2016-01040)References
1. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease - Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84.
2. Goceri E, Shah ZK, Layman R, et al. Quantification of liver fat: A comprehensive review. Computers in Biology and Medicine. 2016;71:174–89.
3. Hernando D, Sharma SD, Aliyari Ghasabeh M, et al. Multisite, multivendor validation of the accuracy and reproducibility of proton-density fat-fraction quantification at 1.5T and 3T using a fat-water phantom. Magn Reson Med. 2017;77(4):1516–24.
4. Kang B, Kim M, Song S, et al. Feasibility of modified Dixon MRI techniques for hepatic fat quantification in hepatic disorders: validation with MRS and histology. Br J Radiol. 2017;12.
5. Berglund J, Kullberg J. Three-dimensional water/fat separation and T2* estimation based on whole-image optimization-Application in breathhold liver imaging at 1.5 T. Magn Reson Med. 2012;67(6):1684–93.
6. Yu H, Shimakawa A, Hines CDG, et al. Combination of complex-based and magnitude-based multiecho water-fat separation for accurate quantification of fat-fraction. Magn Reson Med. 2011;66(1):199–206.
7. Hernando D, Hines CDG, et al. Addressing phase errors in fat-water imaging using a mixed magnitude/complex fitting method. Magn Reson Med. 2012;67(3):638–44. 8. Klein S, Staring M, Murphy K, et al. Elastix: A Toolbox for Intensity-Based Medical Image Registration. IEEE Trans Med Imaging. 2010;29(1):196–205.
9. Klein S, Pluim JPW, Staring M, et al. Adaptive Stochastic Gradient Descent Optimisation for Image Registration. Int J Comput Vis. 2008;81(3):227.
10. Yan Z, Zhang S, Tan C, et al. Atlas-based liver segmentation and hepatic fat-fraction assessment for clinical trials. Comput Med Imaging Graph. 2015;41:80–92.
11. Zheng Y, Georgescu B, Ling H, et al. Constrained marginal space learning for efficient 3D anatomical structure detection in medical images. IEEE, CVPR Workshops. 2009:194–201.
12. Christ PF, Elshaner MEA, Ettlinger F, et al. Automatic Liver and Lesion Segmentation in CT Using Cascaded Fully Convolutional Neural Networks and 3D Conditional Random Fields. Proc 19th Int Conf Med Image Comput Comput Assist Interv. 2016;1–8.
Figures