4721
Rapid and noninvasive detection and dynamic quantification of gut bleeds with Magnetic Particle Imaging1Department of Bioengineering, University of California, Berkeley, CA, United States, 2Lodespin Labs, Seattle, WA, United States, 3Magnetic Insight, Inc., Alameda, CA, United States, 4Department of Material Science and Engineering, University of Washington, Seattle, WA, United States, 5Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 6University of San Francisco Medical Center, San Francisco, CA, United States, 7Department of Electrical Engineering and Computer Sciences, University of California, Berkeley, CA, United States
Synopsis
Magnetic Particle Imaging (MPI) is a novel, high-contrast, and quantitative imaging modality that directly detects superparamagnetic iron oxide nanoparticle (SPIO) tracers. These SPIOs have been previously used as a MRI contrast agent. However, with MRI SPIOs are limited by poor specificity and difficulty associated with quantifying the negative signal. Due to its direct detection, high sensitivity and positive contrast, MPI is uniquely poised as a clinically translatable platform for vascular imaging, including gastrointestinal (GI) bleed detection. Here we present in vivo GI bleed detection using long-circulating SPIOs as the vascular agent in a mouse model of Familial Adenomatous Polyposis.
Introduction:
Gastrointestinal (GI) bleeds can occur spontaneously, after physical trauma or as a result of surgical complications, and are responsible for approximately 300,000 hospitalizations in the US.1 Currently, diagnostic techniques for GI bleeds rely on Nuclear Medicine (NM), CT Angiography (CTA) and MR Angiography (MRA). The NM technique to track red blood cells (RBCs) tagged with Tc99m is commonly the first test, because it is the most sensitive for detecting very slow bleeds. However, tradeoffs include radioactive dose to the patient, poor spatial resolution (~4 mm), bulky radio-pharmaceutical agent preparation and long scan times, which can delay surgical interventions. Long circulating Super-paramagnetic Iron Oxide nanoparticles (SPIO) present a biocompatible and safe alternative to radioactive tracers. SPIOs have been used and characterized in research as a contrast agent for MRI. However, the negative image contrast produced limits clinical translation and the accuracy of quantification. Here, we present a new imaging modality called Magnetic Particle Imaging (MPI), which is capable of directly detecting SPIO to detect GI Bleeds in a mouse model of Familial Adenomatous Polyposis.Materials and Methods:
Twelve week old C57BLK6/APCmin/+ mice (n = 5) with a genetic mutation in FAP (JAX® Laboratory) (Hct=0.21-0.30) were scanned using MPI.2 Wild-type C57BL/6 mice (n=3) were used as a control. MPI was carried out with a home built vertical bore field-free line (FFL) MPI scanner with a gradient strength of 6.3 T/m. Dynamic projection MPI scans were acquired with respiratory gating (21 scans over 86 minutes). SPIOs (LodeSpin-017, 5 mg of Fe/Kg,100 μL) were injected through a lateral tail vein catheter prefilled with heparin diluted with 1X PBS. MPI data was obtained as 2D projection over 120 minutes. For improved contrast, the first time point was digitally subtracted from each of the time courses, with negative values set to zero to capture positive tracer accumulation. Region-Of-Interest based flow quantification was performed after converting the MPI signal to iron concentration.Results and Discussion
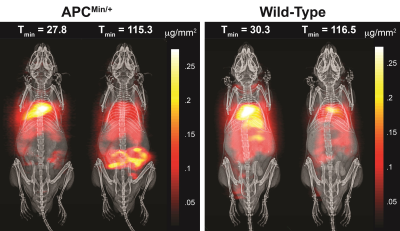
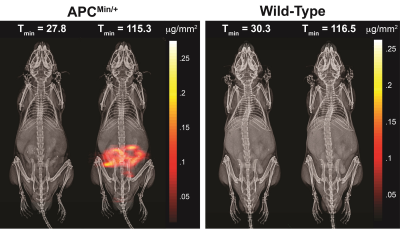
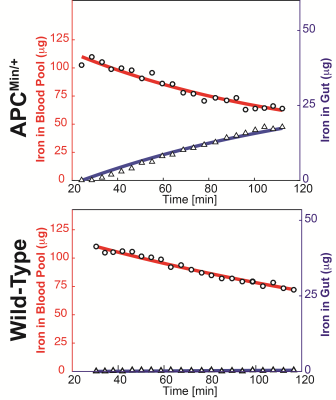
2D MPI projection image of C57BLK6/APCmin/+ and C57BL6 mice 30 min and 120 minutes post-SPIO injection are shown in Figure 1. Subtracted images were used to visualize positive tracer accumulation, as seen in Figure 2. MPI signal was observed in the mouse colon of the FAP model, whereas the wild-type showed signal from the vascular compartment, typical of LS-017 (a blood pool tracer). The tracer accumulation of the gut lumen was linear with time and an average flow rate of 4.25 μL/min (Figure 3). In addition, ex vivo and in vivo MPI signal were compared for all mice, and a near unity slope of 0.98 was obtained for the linearity fit (r2 = 0.98). This demonstrates the quantitative nature of MPI. Currently, 99mTc-labelled RBC is the gold-standard for detecting active GI bleeds over a long period.3 In this work, we were able detect gut bleeds with high sensitivity by using a long circulating, non-radioactive SPIO tracer. Future work could focus on the advantages provided by multi-modality imaging with co-registered MPI and MRI.Acknowledgements
The authors would like to acknowledge funding support from NIH 5R01EB019458-03, NIH5R24MH106053-03, UC Discovery Grant 29623, W. M. Keck Foundation Grant 009323, and NSF GRFP for this work. Additionally, work at Lodespin Labs and University of Washington was supported by NIH 1R41EB013520-01 and NIH 2R42EB013520-02A1.References
[1] B. S. M. Kim, B. T. Li, A. Engel, J. S. Samra, S. Clarke, I. D. Norton and A. E. Li, World Journal of Gastrointestinal Pathophysiology, vol. 5, pp. 467, 2014.gi
[2] H. Wei, J. Shang, C. Keohane, M. Wang, Q. Li, W. Ni, K. O'Neill and M. Chintala, Thromb. Haemost., vol. 111, pp. 1121-1132, 2014.
[3] Graça BM, Freire PA, Brito JB, Ilharco JM, Carvalheiro VM, Caseiro-Alves F. , Radiographics : a review publication of the Radiological Society of North America, Inc. 2010;30(1):235-52.
Figures