4715
Evaluating Response of Locally Advanced Gastric Adenocarcinoma to Neoadjuvant Chemotherapy using Intravoxel Incoherent Motion MRI: A Preliminary study1Department Of Imaging Diagnosis, National Cancer Center / Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China, 2GE healthcare, China, Beijing, China
Synopsis
Intravoxel incoherent motion (IVIM) diffusion-weighted magnetic resonance imaging (DW-MRI) has been applied in research of different cancers, however its potential in gastric cancer has not been fully explored. In this study, we explored the value of IVIM parameters in evaluating the response to chemotherapy in gastric cancer. We found that the D and f values showed good diagnostic performance by differentiating responders from nonresponders, this could provide effective help for the choice of clinical treatment.
Purpose
To investigate the value of IVIM DW-MRI in evaluating the response to neoadjuvant chemotherapy (NCT) in locally advanced gastric adenocarcinoma (LAGA).Methods
Twenty-nine stage II–IVa LAGA patients diagnosed between 2014 and 2016 in the National Cancer Center of China were selected. All patients received 3-6 cycles of SOX NCT, and underwent two IVIM DW-MRI studies using 10 different b values (b=0, 10, 20, 50, 100, 200, 400, 600, 800, 1200 s/mm2) on a 3.0-Tesla MRI scanner (GE Discovery MR750 with an 8-channel CTL Target Array Coil): a baseline scan before therapy and a post-treatment scan within 2 weeks after NCT finished. Diffusion coefficient (D), perfusion fraction (f) and pseudo-diffusion coefficient (D*) maps were calculated from the bi-exponential model. IVIM parameters (D, D*, and f) of LAGC were measured by region-of-interest (ROI) methods using the FuncTool on GE AW4.6 workstation. All patients received radical resection within 2 weeks after the second examination. According to the Mandard pathologic tumor regression grade (TRG), subjects were divided into responders (TRG 1-3) and nonresponders (TRG 4, 5). The IVIM parameters before (pre-parameters) and after (post-parameters) NCT and their corresponding changes (Δparameters) between the two groups were compared using the Student’s t test or nonparametric test. The diagnostic performance of different parameters was judged by the receiver-operating characteristic curve (ROC) analysis.Results
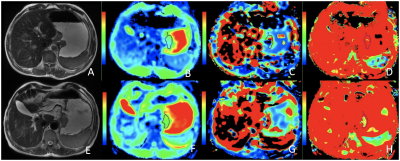
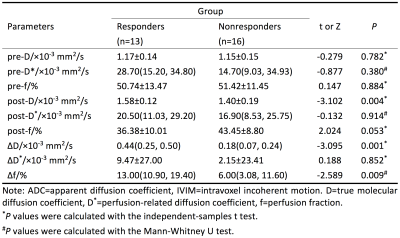
Based on the Mandard TRG criteria after 4-6 cycles of NCT, 13 patients were categorized into the responder group (Figure 1) whereas the other 16 patients were considered nonresponders(Figure 2). The D value was significantly higher after treatment and the f value was significantly lower (all P < 0.05) (Table 1). In contrast, D* value was only slightly lower after treatment (P > 0.05). Compared with nonresponders, a notably higher post-D value, ΔD and Δf were observed in responders (all P < 0.05), but no significant change was observed for other parameters among the 2 groups (P > 0.05) (Table 2). The ROC curve analysis indicated that the cutoff of ΔD value in best predicting TRG was 0.42×10-3 mm2/s (Table 3), and the corresponding AUC, sensitivity, and specificity were 0.841, 66.7%, and 100.0%, respectively.Discussion
Predicting tumor response to chemotherapy as accurate as possible could help determine an optimal treatment regimen and have considerable clinical benefits for LAGA patients. But now there is no widely accepted curative effect evaluation standard of NCT in gastric cancer. The RECIST, a widely adopted standard for evaluating therapy response based on the change in tumor size, is restricted in gastric cancer, since the stomach is a cavity viscera. Recently, IVIM DW-MRI showed promising potential for noninvasive assessment of curative effects in a variety of tumors. In this study, post-D value and ΔD which represent the true molecular diffusion were significant higher in responders, reflected a more decreased tumor cell density and the restrictions of water molecules in this group. We found the f value significantly decreased after NCT and Δf was significantly higher in responders, maybe due to a decreased microvasculature, blood flow velocity and vessel architecture. But the D* value, due to its poor reproducibility, showed no significant different after NCT and between the two groups.Conclusion
IVIM-derived parameters, especially the D and f values, showed potentials in the prediction and response monitoring of neoadjuvant chemotherapy in LAGA.Acknowledgements
No acknowledgement found.References
[1] Newton AD, Datta J, Loaiza-Bonilla A, et al. Neoadjuvant therapy for gastric cancer: current evidence and future directions. J Gastrointest Oncol. 2015 Oct;6(5):534-43.
[2] Desar IM, van Herpen CM, van Laarhoven HW, et al. Beyond RECIST: molecular and functional imaging techniques for evaluation of response to targeted therapy. Cancer Treat Rev. 2009 Jun;35(4):309-21.
[3] Hallinan JT, Venkatesh SK. Gastric carcinoma: imaging diagnosis, staging and assessment of treatment response. Cancer Imaging. 2013 May 30;13:212-27.
[4] Tang L, Sun YS, Li ZY, et al. Diffusion-weighted magnetic resonance imaging in the depiction of gastric cancer: initial experience. Abdom Radiol (NY). 2016 Jan;41(1):2-9.
[5] Iima M, Le Bihan D. Clinical Intravoxel Incoherent Motion and Diffusion MR Imaging: Past, Present, and Future. Radiology. 2016 Jan;278(1):13-32.
[6] Koh DM. Science to practice: can intravoxel incoherent motion diffusion-weighted MR imaging be used to assess tumor response to antivascular drugs? Radiology. 2014 Aug;272(2):307-8.
[7] Zhu L, Zhu L, Shi H. Evaluating early response of cervical cancer under concurrent chemo-radiotherapy by intravoxel incoherent motion MR imaging. BMC Cancer. 2016 Feb 10;16:79.
Figures