4708
High-Resolution Intravoxel Incoherent Motion (IVIM) diffusion-weighted MR imaging for the characterization of periampullary lesions1Department of Radiology, People’s Hospital of Zhengzhou University, Henan Provincial People’s Hospital, Zhengzhou, China, 2MR Application Predevelopment, Siemens Healthcare, Erlangen, Germany, 3Siemens Healthcare, MR Collaborations NE Asia, Beijing, China
Synopsis
This study aimed to investigate the clinical diagnostic utility of ZOOMit-DWI-based IVIM imaging technology[1,2] for periampullary lesions. Forty-one patients, comprising cancer of the pancreatic head (n = 6), chronic pancreatitis (n = 9), ampullary adenocarcinoma (n = 9), and distal bile duct carcinoma (n = 17) and 28 healthy volunteers were enrolled. Our results showed that the perfusion fraction (f) of the IVIM-derived parameter has the potential to distinguish between cancer of the pancreatic head, other lesions, and normal tissue in the periampullary regions by using the zoomed DW imaging, which has the capacity to overcome some of the limitations of conventional MRI of the pancreas.
Background and Purpose
There are several causes of obstructive jaundice (OJ), and the periampullary lesions are a significant one. Accurate positioning and qualitative diagnosis are essential for the development of treatment regimens, the choice of a surgical approach, and the prediction of prognosis[3]. Due to the large FOV for abdominal imaging and field inhomogeneity[1,2], accurate diagnosis is difficult to achieve in the ampulla of Vater, and conventional diffusion-weighted MR images do not have a sufficient spatial resolution in this type of application. The conventional DW image of the ampulla of Vater is seriously deformed and the ADC value is unstable[1], resulting in relatively low diagnostic accuracy. In this study, ZOOMit-DWI was used to achieve high-resolution DW images. Furthermore, an intravoxel incoherent motion (IVIM) model was applied to demonstrate not only water molecular diffusion but also microcirculation of different origins and the nature of the periampullary lesions[4-9]. Therefore, it is possible to obtain more information and test the clinical application value of ZOOMit-DWI in periampullary lesions.Materials and Methods
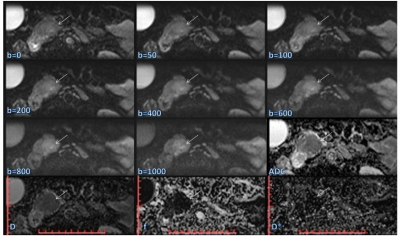
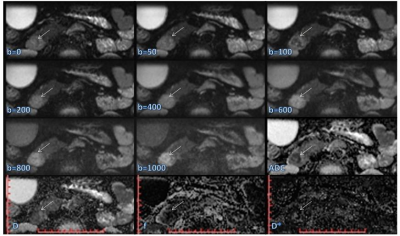
This prospective study was approved by our hospital ethics committee. All the healthy volunteers and patients provided written informed consent before enrollment. Between August 2016 and August 2017, a total of 41 patients, including those with cancer of the pancreatic head (pancreatic adenocarcinoma, n = 6; 2 males; age, 61.5 ± 8.4 years; age range, 52 - 70 years), chronic pancreatitis (n = 9; 5 males; age, 47.9 ± 16.9 years; age range, 28 - 78 years), ampullary adenocarcinoma (n = 9; 4 males; age, 59.0 ± 7.9 years; age range, 48 - 73 years), and distal bile duct carcinoma (n = 17; 10 males; age, 64.0 ± 7.8 years; age range, 53 - 75 years) were enrolled in this study before surgery or biliary interventional procedures were performed. During the same period, 28 healthy volunteers (16 males; age, 64.0 ± 7.8 years; age range, 53 - 75 years) also underwent MR examinations as the control group. All the MRI examinations were performed using a MAGNETOM Prisma 3T MR Scanner (Siemens Healthcare, Erlangen, Germany) with an 18-channel body coil and 32-channel spine coil. All the participants underwent a contrast-free MR exam, including magnetic resonance cholangiopancreatography (MRCP) and ZOOMit-IVIM DWI. The DWI were acquired by applying a ZOOMit-EPI sequence in free breathing with 8 b-values (0, 50, 100, 200, 400, 600, 800, and 1000 sec/mm2). The primary parameters of the ZOOMit-IVIM DWI were as follows: TR = 3500 ms, TE = 60 ms, field of view = 200*45 mm2, matrix = 120 x 54, slice thickness = 4 mm, number of slices = 18, voxel size = 1.7*1.7*4 mm3, and TA = 3:39 min. The apparent diffusion coefficient (ADC), the true diffusion constant (D), pseudo-diffusion coefficient (D*), and perfusion fraction (f) were calculated using the Body Diffusion Toolbox prototype software (Siemens Healthcare, Erlangen Germany). These parameters were subsequently compared using the Mann-Whitney U and Kruskal-Wallis tests.Results
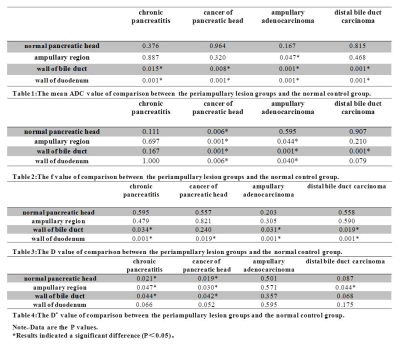
Among all the parameters derived from both the IVIM and standard DWI model, there were no significant differences between the head, body, and tail of the normal pancreas (P > 0.05). The f value of cancer of the pancreatic head was significantly lower than that of chronic pancreatitis, ampullary adenocarcinoma, and distal bile duct carcinoma (P < 0.05). For differentiation of the periampullary lesion groups from the normal control group, the details of the ADC, f, D, and D* are shown in Tables 1, 2, 3 and 4(Figure 1), respectively. The D* value of chronic pancreatic cancer (P < 0.05) and the f value of cancer of pancreatic head (P < 0.01) were used for differentiating from the normal pancreatic head, ampullary region, and the wall of the bile duct and duodenum.Discussion and Conclusion
The results of this study showed that ZOOMit-DWI has the potential to overcome some of the limitations of conventional EPI techniques in performing MRI of the pancreas. Moreover, the perfusion fraction (f) of the IVIM-derived parameter was helpful for the differentiation of cancer of the pancreatic head and other lesions and normal tissue in the periampullary regions.
Acknowledgements
No acknowledgement found.References
1. Thierfelder KM, Sommer WH, Dietrich O, et al.: Parallel-transmit-accelerated spatially-selective excitation MRI for reduced-FOV diffusion-weighted-imaging of the pancreas. European journal of radiology 2014;83:1709-1714.
2. Samson RS, Levy S, Schneider T, et al.: ZOOM or Non-ZOOM? Assessing Spinal Cord Diffusion Tensor Imaging Protocols for Multi-Centre Studies. PloS one 2016;11:e0155557.
3. Kim JH, Kim MJ, Chung JJ, et al.: Differential diagnosis of periampullary carcinomas at MR imaging. Radiographics : a review publication of the Radiological Society of North America, Inc 2002;22:1335-1352.
4. Barral M, Taouli B, Guiu B, et al.: Diffusion-weighted MR imaging of the pancreas: current status and recommendations. Radiology 2015;274:45-63.
5. Kartalis N, Manikis GC, Loizou L, et al.: Diffusion-weighted MR imaging of pancreatic cancer: A comparison of mono-exponential, bi-exponential and non-Gaussian kurtosis models. European Journal of Radiology Open 2016;3:79-85.
6. Ma C, Li Y, Wang L, et al.: Intravoxel incoherent motion DWI of the pancreatic adenocarcinomas: monoexponential and biexponential apparent diffusion parameters and histopathological correlations. Cancer imaging : the official publication of the International Cancer Imaging Society 2017;17:12.
7. Kamisawa T, Takuma K, Anjiki H, et al.: Differentiation of autoimmune pancreatitis from pancreatic cancer by diffusion-weighted MRI. The American journal of gastroenterology 2010;105:1870-1875.
8. Sandrasegaran K, Nutakki K, Tahir B, et al.: Use of diffusion-weighted MRI to differentiate chronic pancreatitis from pancreatic cancer. AJR American journal of roentgenology 2013;201:1002-1008.
9. Koh DM, Collins DJ, Orton MR: Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR American journal of roentgenology 2011;196:1351-1361.
Figures