4699
Placental Insufficiency Investigated with Multi-compartment Placental MRI1University College London, London, United Kingdom, 2University College Hospital, London, United Kingdom
Synopsis
Efficient exchange of oxygen and nutrients across the placenta is vital for a normally grown fetus. When remodelling of maternal arteries does not occur in early pregnancy the result is placental insufficiency, and fetal growth restriction. Previous studies have shown differences in T2 relaxometry and IVIM between normal and FGR placentae. Here we use a combined T2R and IVIM signal model that separates signals from fetal and maternal blood pools over the whole placental volume. We show difference in T2R, ADC, and maternal perfusion fraction, findings in keeping with previous literature and the pathophysiology of placental insufficiency.
Introduction
Efficient exchange of oxygen and nutrients across the placenta is vital for a normally grown fetus. One third of stillbirths are caused by placental insufficiency1, but the vascular properties of the placenta cannot be accurately measured in utero and remain poorly understood. Trophoblast (placental cell) remodelling of the maternal spiral arteries in the decidua normally results in a high volume low resistance maternal placental circulation2. This remodelling is inadequate in placental insufficiency4 and will lead to fetal growth restriction (FGR). There is no treatment that can improve placental perfusion, thus the only intervention is careful planning of early delivery, balancing the risk of fetal demise due to placental failure against the risks of severe prematurity of birth. Previous placental MRI studies of T2 relaxometry (T2R) and Intravoxel incoherent motion (IVIM)4,5, have shown differences between normal and FGR placentae6,7. Here we use a combined T2R and IVIM signal model10 that separates signals from fetal (f*) and maternal (v) blood pools over the whole placental volume, and use this to investigate fetal and maternal placental perfusion in normal singleton pregnancies and FGR.Methods
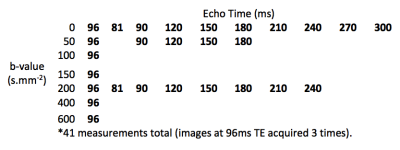
Four women in mid-pregnancy (28+4 to 34+0 gestational weeks) with normally grown fetuses, and three with severe FGR (25+4 to 28+5 gestational weeks) consented for fetal MRI. Obstetric ultrasound confirmed normal fetal growth and fetal and maternal Doppler studies in the control group, all of whom delivered a normally grown baby at term. Ultrasound in the FGR group found estimated fetal weight less than the 1st centile in all cases, with increased resistance in the uterine artery Doppler’s. FGR1 had normal umbilical artery Doppler’s, and delivered at full term whilst FGR2 and FGR3 had abnormal umbilical artery Doppler’s and delivered at 29+5 and 28+6 gestational weeks respectively. Imaging was performed on 1.5T Siemens Symphony, taking 41 measurements grouped across 7 b-values and 10 echo times (FIGURE 1). We adapt the DECIDE model10 to take into account typical T2 values from recent literature11 and assign a fetal T2 of 190ms and a maternal T2 of 230ms based upon estimated oxygen saturations11,12.Results
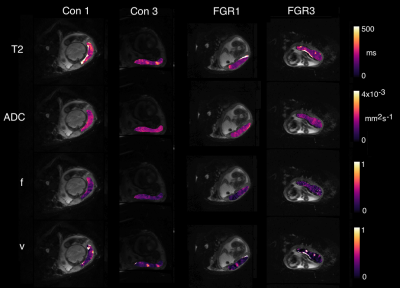
Figure 2 shows example parametric maps for two cases of FGR and two control pregnancies. T2 and ADC maps are visually quite similar between control and pathological placenta, whilst volume fractions of fetal (f*) and maternal (v) perfusion are visibly reduced.
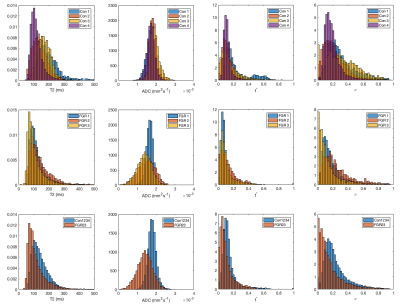
Figure 3 shows parametric histograms over the whole placenta for four imaging parameters. ADC distributions in column two are observed to be different between control and FGR placentae with mean ADC 0.0018(0.0002)mm2s-1 for control and ADC=0.0014(0.004)mm2s-1 for FGR2/3 (95% CI: [-0.0004,-0.0004]mm2s-1). Interestingly the FGR case that delivered at term has an ADC closer to the appearance of the control placentae (ADC=0.0017(0.0002) mm2s-1), motivating its removal from the reported FGR statistics. T2 was reduced in FGR (143.7(59)ms normal vs 119.7(68)ms FGR2/3 (95% CI: [-24.9,21.6]ms)). Fetal perfusion fraction (f*) was similar (0.14(0.11) control vs 0.11(0.12) FGR2/3 (95% CI: [-0.034,-0.028])), whilst the maternal perfusion fraction (v) was reduced in FGR (0.24(0.16) control vs 0.17(0.17) FGR2/3 (95% CI: [-0.075,-0.065])).
Conclusion
Fetal Growth Restriction shows marked differences from control imaging data on multi-parametric MRI. Average T2 and ADC values are shown to be lower, replicating previous results in FGR, whilst the interpretation of these results as vascular volume fractions allows the observation that FGR cases have reduced maternal perfusion fraction (v), in keeping with the known pathophysiology of placental insufficiency.Acknowledgements
This work was supported through an Innovative Engineering for Health award by the Wellcome Trust [ WT101957 ]; Engineering and Physical Sciences Research Council (EPSRC) [ NS/A000027/1 ], and the Wellcome/EPSRC Centre EPSRC [ NS/A000050/1 ] Wellcome [ 203145Z/16/Z ].References
1. Lawn, J. E. et al. Stillbirths: Where? When? Why? How to make the data count? Lancet (London, England) 377, 1448–63 (2011).
2. Fox, H. & Sebire, N. J. Pathology of the Placenta. (Saunders Elsevier, 2007).
3. Fox, H. THE PATTERN OF VILLOUS VARIABILITY IN THE NORMAL PLACENTA. BJOG An Int. J. Obstet. Gynaecol. 71, 749–758 (1964).
4. Moore, R. J. et al. In vivo intravoxel incoherent motion measurements in the human placenta using echo-planar imaging at 0.5 T. Magn. Reson. Med. 43, 295–302 (2000).
5. Gowland, P. A. et al. In vivo relaxation time measurements in the human placenta using echo planar imaging at 0.5 T. Magn. Reson. Imaging 16, 241–7 (1998).
6. Derwig, I. et al. Association of placental perfusion, as assessed by magnetic resonance imaging and uterine artery Doppler ultrasound, and its relationship to pregnancy outcome. Placenta 34, 885–91 (2013).
7. Derwig, I. et al. Association of placental T2 relaxation times and uterine artery Doppler ultrasound measures of placental blood flow. Placenta 34, 474–9 (2013).
8. Moore, R. J. et al. In utero perfusing fraction maps in normal and growth restricted pregnancy measured using IVIM echo-planar MRI. Placenta 21, 726–32 (2000).
9. Capuani, S. et al. Diffusion and perfusion quantified by Magnetic Resonance Imaging are markers of human placenta development in normal pregnancy. Placenta 58, 33–39 (2017).
10. Melbourne, A. et al. DECIDE: Diffusion-rElaxation Combined Imaging for Detailed Placental Evaluation. ISMRM 25th Annu. Meet. Exhib. (2017).
11. Portnoy, S. et al. Relaxation Properties of Human Umbilical Cord Blood at 1.5 Tesla. Magnetic Resonance in Medicine 77:1678–1690 (2017)
12. Murphy P.J. The fetal circulation. Continuing Education in Anaesthesia Critical Care & Pain, Volume 5, Issue 4, 1 August 2005, Pages 107–112.
Figures