4687
Diffusion kurtosis imaging for the diagnosis and prediction of response to treatment in high grade serous ovarian cancer1Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 2Addenbrooke's Hospital, Cambridge, United Kingdom, 3Cancer Research UK, Cambridge, United Kingdom
Synopsis
Diagnosis of high grade serous ovarian cancer (HGSOC) requires a biopsy which is both invasive and does not reflect the heterogeneity of the disease. Diffusion kurtosis imaging (DKI) was performed on 23 treatment naïve ovarian cancer patients. Both mean Dapp (apparent diffusion) and mean Kapp (apparent kurtosis) were found to be significantly different in HGSOC compared to other types of epithelial cancer. Kapp was also significantly greater in the patients who went on to respond to chemotherapy treatment compared to non-responders. DKI may therefore aid in identifying HGSOC and in the selection of the best treatment for individual patients.
Introduction
Ovarian cancer prognosis depends on the cancer subtype and stage at diagnosis. 90% of ovarian cancers are epithelial in nature with high grade serous ovarian cancer (HGSOC) having the highest mortality. The best outcomes in HGSOC occur when treatment is with a combination of surgery and chemotherapy [1] but there is currently no imaging or serum biomarker test that can reliably separate HGSOC from other epithelial cancers. A non-invasive imaging test to stratify patients with ovarian cancer would aid both diagnosis and treatment response assessment. This research describes the use of diffusion kurtosis imaging (DKI): an advanced form of diffusion weighted imaging, to probe the non-Gaussian movement of water in the ovarian cancer tumor microenvironment. We also show here how DKI can be used as a biomarker to predict response to chemotherapy treatment.Methods
Single-shot echo planar diffusion weighted imaging was performed on 23 patients with ovarian tumors using a 3T Discovery MR750 (GE Healthcare, Waukesha WI) scanner with a 32-channel cardiac array and 4 signal averages. Scan parameters were: TR 6000ms, TE 94ms, flip angle 90°, slice thickness 6mm, FoV 34.0 cm × 29.9 cm, matrix size 128 × 112, b-values 100, 500, 900, 1300 and 1700 s/mm2. Dapp (apparent diffusion) and Kapp (apparent kurtosis) maps were calculated by performing a pixelwise non-linear fit to the bi-exponential diffusion model described in equation (1) below,
$$ S(b) = S0·exp (−b· Dapp + 1/6 · b2 · Dapp2 · Kapp) $$
with additional corrections to compensate for the effects of the noise floor using a noise-only image. Fit failure pixels were excluded from analysis.
Cancers were stratified by histopathological diagnosis into HGSOC and other epithelial cancers. Primary ovarian and peritoneal lesions were analysed separately. HGSOC patients were further subdivided into responders and non-responders based on tumor bulk measured by contrast enhanced CT before and after 3 cycles of chemotherapy. Response was defined as a decrease in disease volume of 30% or more. Statistics were performed using R (version 2.15.3, R Foundation for Statistical Computing, Vienna, Austria). The t-test or Wilcoxon signed-rank test was used as appropriate to test for differences between groups at a significance level of 0.05. Lesion ROIs were checked by a gynecological radiologist.
Results
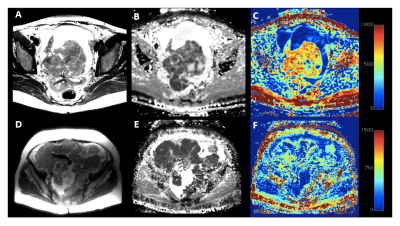
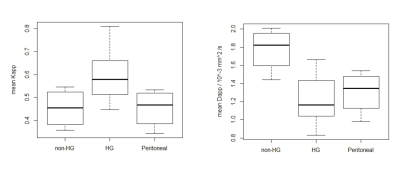
Figure 1 shows typical Dapp and Kapp maps produced for primary ovarian and peritoneal lesions. Pixels from the primary ovarian lesions fit the DKI model well (9% fit failure) but for the peritoneal lesions there was a 28% fit failure. Figure 2 gives the mean Dapp and Kapp values for the different lesion types and treatment outcomes analysed. Figure 3 shows boxplots of Kapp and Dapp for the different tumor subtypes. Kapp was significantly greater for HGSOC than for other tumor subtypes p<0.05, while Dapp was significantly lower in HGSOC p<0.01. There was a significant difference in the pre-chemotherapy mean Kapp of the patients who ultimately responded to chemotherapy compared to the group who did not respond; p< 0.005. A similar analysis for Dapp did not show a significant difference between the responder and non-responder groups with a p-value of 0.14. A box-plot of the mean Dapp and Kapp values for responders and non-responders is shown in Figure 4.Discussion
The magnitude of Kapp reflects the extent to which the movement of water molecules deviates from a Gaussian model. Kapp may therefore relate to cellular heterogeneity or the level of complexity in the tissue microstructure. HGSOC was predicted to have a higher Kapp than lower grade ovarian tumors due to the more heterogeneous appearance on histology of HGSOC tumours, and this was confirmed in this study. Dapp was also shown to be higher in HGSOC tumors which is consistent with these tumors having greater cellularity and heterogeneous restriction to diffusion. DKI has previously been demonstrated to predict neoadjuvant chemotherapy response in other cancers [2]. Results from this study indicate that the same may be possible for HGSOC. Further work is needed to verify the results found here in a larger cohort.Conclusion
Higher grade ovarian lesions have a worse prognosis and require different treatment approaches compared to lower grade tumors [3]. Here we show that tumor Kapp could provide additional information to improve the diagnostic confidence in confirming HGSOC. Importantly, it was also shown that Kapp may be able to identify which HGSOC patients will respond successfully to chemotherapy.Acknowledgements
The authors would like to acknowledge support for this work from Cancer Research UK, Experimental Cancer Medicine Centres (ECMC), the Gates Cambridge Trust, Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge.References
[1] Vergote, I., et al., Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. New England Journal of Medicine, 2010. 363(10): p. 943-953.
[2] Chen, Y., et al., Diffusion kurtosis imaging predicts neoadjuvant chemotherapy responses within 4 days in advanced nasopharyngeal carcinoma patients. Journal of Magnetic Resonance Imaging, 2015. 42(5): p. 1354-1361.
[3] Vang, R., I.-M. Shih, and R.J. Kurman, Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Advances in anatomic pathology, 2009. 16(5): p. 267.
Figures