4682
MR neurography of the female pelvis: mapping somatic and autonomic nerves and plexi1Institute of Cancer Research, Sutton, United Kingdom, 2Philips, Guildford, United Kingdom, 3Philips, Sydney, Australia, 4The Royal Marsden NHS Foundation Trust, Sutton, United Kingdom
Synopsis
The visualization of somatic and autonomic female pelvic nerves using a modified NerveVIEW protocol was assessed in volunteers (n=5) and cervical cancer patients (n=7) by 2 independent observers. Image quality (as assessed by visualization of the tibial and fibular components of the sciatic nerve) was high in 75% of cases. 83% of pudendal, superior and inferior hypogastric nerves were well seen by observer1 and 72% by observer2. The superior hypogastric plexus (SHP) was more difficult to identify routinely. Neurography of the female pelvis allowed for confident identification of autonomic and somatic nerve plexi and has potential for pre-surgical planning.
Background
Disruption to somatic and autonomic pelvic innervation by a variety of inflammatory and neoplastic pathologies has profound effects namely pelvic pain, incontinence, sexual dysfunction and so critically influences a patient’s quality of life. Delineation of pelvic nerves on MR is challenging, particularly in females where common benign pathologies such as uterine fibroids, and ovarian cysts distort the anatomy. Moreover, flow and pulsation in adjacent vascular structures and bowel peristalsis reduces visualization of complex neural anatomy. We used a heavily T2W sequence [NerveVIEW][1] and modified it for improved visualization of pelvic nerves at 3T.
Aim
To define the somatic (pudendal) and autonomic (superior hypogastric, inferior hypogastric plexi) in healthy volunteers using modified NerveVIEW.Methods
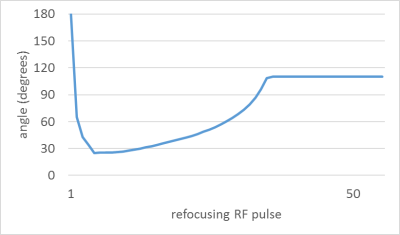
A 3D VIEW pulse sequence was optimized, which provided a 3D large field-of-view (FOV) acquisition with small voxel size. VIEW enables extended echo trains with variable flip angle refocusing sweeps, which preserve magnetization for later in the echo train. The refocusing sweep was tailored to nerve tissue using nominal T1: 1410ms and nominal T2: 82ms (Figure 1). Strong T2-weighting was created with motion-sensitive driven equilibrium (MSDE) preparation to increase nerve contrast, employing two refocusing pulses in a T2prep time of 23.7ms, and flow compensation gradients to suppress signal from veins (VENC 2cm/s). STIR (short TI inversion recovery) was used to suppress fat (inversion time TI 280ms). An important sequence modification which allowed better visualization of pelvic nerves was introduction of an offset independent trapezoid (OIT) pulse [1] for the STIR inversion, and for the MSDE refocusing pulses. The OIT pulses have a larger transmit bandwidth (15kHz) for improved B0 insensitivity over a large FOV versus conventional hyperbolic secant inversion, and in this work yielded improved background fat suppression and T2 contrast. Other pulse sequence details include: acquired resolution: 1.1x1.1x2.0mm; TR,TEequiv: 2300,176ms; TSE factor 51; FOV 324mm; 150 slices, total SENSE acceleration 3.75, scan time 6m33s. 5 healthy volunteers, aged 32-60 years, and 7 women aged 26-62 years with high-grade CIN (n=2) and cervical cancer (Stage 1b n=3, Stage 2b n=2) were imaged with a TorsoXL receiver array on a 3T Philips Achieva using modified NerveVIEW. Maximum Intensity Projections were manipulated on a PACS workstation (observer 1) or a Philips Extended Workstation (observer 2) to enable selection of slab thickness and for manipulation of multiplanar views. Images were scored by each radiologist independently. The quality of the images was rated as high(3), medium(2) or low(1) based on the definition of 9 individual nerve roots within the fibular and tibial trunks of the sciatic nerve. The left & right pudendal nerves, the superior hypogastric plexus (SHP) and left & right inferior hypogastric plexus (IHP) were graded for conspicuity as 0=not seen, 1=poorly seen, 2=well seen.Results
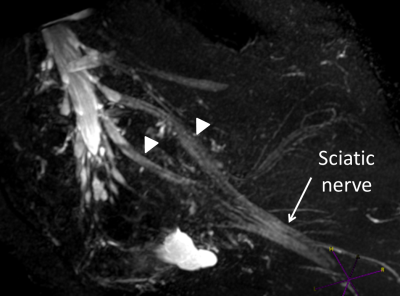
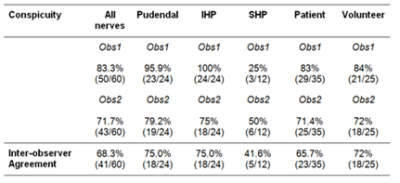
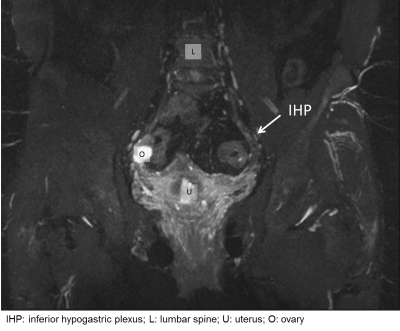
Both observers scored images as medium to high quality in volunteers as well as patients (Figure 2); scores were in agreement in in 75% (9/12)); none of the images were of low quality. Both somatic (pudendal) and autonomic (superior and inferior hypogastric) nerves could be identified in all subjects, except for the SHP in one volunteer, which was not detected by either observer. Considering all nerve groups, 83.3% (50/60) were well seen by observer 1 and 71.7% (43/60) by observer 2. There were no differences between volunteer and patient assessments. Nerves were well seen by observer 1 in 84% (21/25) of volunteers and 83% (29/35) patients; for observer 2 this was 71.4% (25/35) and 72% (18/25) respectively. Observers agreed in their scoring in 72% (18/25) of volunteers and in 65.7% (23/35) of patients. Pudendal nerves were well seen in 95.9% (23/24) by observer1 and 79.2% (19/24) by observer 2; the IHP was well seen in 100% (24/24) by observer 1 and 75% (18/24) by observer 2 (Figure 3). The SHP was well seen in 25% (3/12) by observer 1 and poorly seen in 66.7% (8/12), while observer 2 identified the SHP well in 50% (6/12) and poorly in 41.7% (5/12). Both observers did not detect the SHP in one case (8.3%).Observers agreed in their conspicuity assessments in 75.0% (18/24) of pudendal and IHP nerves and in 41.6% (5/12) of SHP nerves.Conclusion
Good quality MR neurography was attained using a modified NerveVIEW protocol, enabling visualization of the female pelvic plexi in all volunteers and early stage cervical cancer patients. Conspicuity of the SHP was lower than conspicuity of pudendal nerve and inferior hypogastric plexus, which were well seen in the majority of cases. Further studies are needed to demonstrate the potential of MR neurography for pre-surgical planning as well as for visualization and monitoring radiotherapy-induced nerve damage.
Acknowledgements
CRUK and EPSRC support to the Cancer Imaging Centre at ICR and RMH in association with MRC and Department of Health C1060/A10334, C1060/A16464 and NHS funding to the NIHR Biomedical Research Centre and the Clinical Research Facility in Imaging.References
[1] A. Tannús and M. Garwood, “Adiabatic pulses.,” NMR Biomed., vol. 10, no. 8, pp. 423–34, Dec. 1997.Figures