4681
Developing and validating a multivariable prediction model to improve the diagnostic accuracy in determination of cervical vs. endometrial origin of uterine adenocarcinomas: A prospective MR study combining diffusion-weighted imaging and spectroscopy1Medical Imaging and Intervention, Chang Gung Memorial Hospital, Linkou, Taiwan, 2Graduate Institute of Applied Physics, National Chengchi University, Taipei, Taiwan, 3Gynecology Oncology, Chang Gung Memorial Hospital, Linkou, Taiwan
Synopsis
We developed and validated an MDS score based on integrated morphological, volumetric DW MR imaging and spectroscopy which has incremental values and may be a useful clinical biomarker in distinguishing adenocarcinomas of cervical or endometrial origin.
Purpose
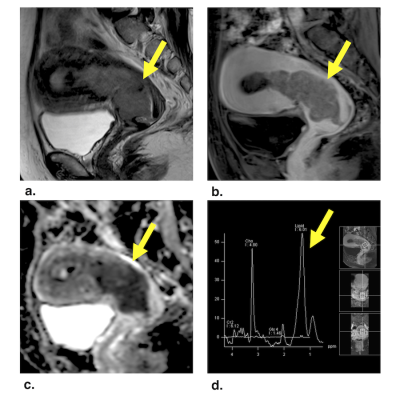
To develop and validate a multiparametric prediction model based on magnetic resonance (MR) imaging and spectroscopy in distinguishing adenocarcinomas of uterine cervical or endometrial origin.Materials and methods
In the institutional
review board-approved study, we prospectively enrolled 25 cervical and 62
endometrial adenocarcinomas for 3.0-T MR staging. We developed an MDS score
based on the Morphological traits, volumetric apparent diffusion
coefficient (ADC) analysis on diffusion-weighted (DW) MR imaging,
and single-voxel MR Spectroscopy from the training dataset (n = 43). The
predictive values of MDS score were validated independently (n = 44).
Data were analyzed using logistic regression and receiver operator
characteristic (ROC) curves analysis.Results
We constructed a 5-point MDS score based on the tumor ADCmean, fatty acyl δ 1.3 ppm, and morphological traits − epicenter at the cervix, rim enhancement, and disrupted cervical stromal integrity. In the both the training and validation datasets, the MDS score achieved an accuracy of 93.0% and 84.1%, significantly higher than that of morphology (88.4% and 79.5%), ADC value (74.4% and 68.2%) and spectroscopy (81.4% and 68.2%, P < .05 for all). The performances of the MDS score were significantly superior to the morphological traits in the training dataset (AUC = 0.95 v 0.89, P = .046), with a similar trend in the validation dataset (AUC = 0.90 v 0.85, P = .289).Conclusion
The MDS score based on MR DW imaging and spectroscopy has potentials in precisely distinguishing adenocarcinomas of cervical or endometrial origins.Acknowledgements
Supported by Chang Gung Medical Foundation grant CIRPG3E0023, National Science Council (Taiwan) MOST 104-2314-B-182A-095-MY3. Chang Gung IRB 102-0620A3, IRB 103-7316A3. The authors acknowledge the assistance provided by the Clinical Trial Center, Chang Gung Memorial Hospital, Linkou, Taiwan, which was founded by the Ministry of Health and Welfare of Taiwan MOHW106-TDU-B-212-113005.References
1. Vargas HA, Akin O, Zheng J, et al. The value of MR imaging when the site of uterine cancer origin is uncertain. Radiology 2011;258(3):785-792.
2. Lin YC, Lin G, Chen YR, Yen TC, Wang CC, Ng KK. Role of magnetic resonance imaging and apparent diffusion coefficient at 3T in distinguishing between adenocarcinoma of the uterine cervix and endometrium. Chang Gung Med J 2011;34(1):93-100.
3. Lin G, Lai CH, Tsai SY, et al. 1 H MR spectroscopy in cervical carcinoma using external phase array body coil at 3.0 Tesla: Prediction of poor prognostic human papillomavirus genotypes. J Magn Reson Imaging 2017;45(3):899-907.
Figures