4679
Clinical utility of susceptibility-weighted MR sequence (SWAN) for the evaluation of uterine sarcomas1Department of Radiology, Tokushima University, Tokushima, Japan, 2Department of Radiological Technology, Tokushima Bunri University, Sanuki-city, Japan
Synopsis
Intra-tumoral hemorrhagic necrosis is one of the characteristic pathological finding of uterine sarcomas. High intensity hemorrhagic foci on T1WI may be suggestive finding, however, the prevalence is not high possibly because only methemoglobin could be detected. Signal voids on SWAN may reflect all phases of hemorrhage, especially both deoxyhemoglobin and hemosiderin and could be useful for the diagnosis. Surgically proven ten sarcomas and 22 benign leiomyomas were retrospectively evaluated. High intensity foci on T1WI were detected in four sarcomas (40%) and in none of leiomyomas, whereas signal voids on SWAN were detected in all sarcomas and in one leiomyoma (5%).
Background and purpose of the study
Uterine sarcomas are uncommon heterogeneous group of tumors of mesenchymal, epithelial, or mixed epithelial-mesenchymal origin, and recently show increased incidence possibly due to increase in aging population. Because the clinical course is usually aggressive with poor prognosis, it should be correctly diagnosed for the appropriate surgical management and adjuvant therapy. Various imaging characteristics have been reported based on the morphological appearances, and intra-tumoral necrosis and hemorrhage are suggestive findings for sarcomas1-4). However, both degenerated areas in benign leiomyomas and necrotic areas in sarcomas may appear as unenhanced areas on post-contrast images. Intra-tumoral hemorrhage may appear as high intensity areas on T1WI, however, the prevalence is not high possibly because only methemoglobin in subacute hemorrhage may show high signal intensity. Susceptibility-weighted sequences such as SWI (Susceptibility-weighted imaging) or SWAN (T2 star-weighted angiography) are MR techniques, which maximize sensitivity to susceptibility effects and have exquisite sensitivity to blood products such as deoxyhemoglobin and hemosiderin resulting from acute and chronic to obsolete hemorrhage, respectively5-6). The purpose of this study was to evaluate the capability of susceptibility-weighted sequences in detecting the signal voids due to hemorrhagic necrosis for the diagnosis of uterine sarcomas.Materials and methods
Ten women (mean age 57 years) with pathologically proven uterine sarcomas including six carcinosarcomas, two leiomyosarcomas, one endometrial stromal sarcoma, and one uterine tumor resembling ovarian sex-cord tumors (UTROSCT) who had undergone MRI examinations including SW sequences before surgery were retrospectively evaluated. Gradient-echo T1WI with fat-saturation, and SWAN (2.0) were obtained for all patients with 3-T superconducting MRI system (Discovery MR750, GE Healthcare) with 32-channel body-array torso coils. Two radiologists with 25 and 16 years of experience qualitatively evaluated the images for the presence of signal voids within masses on SWAN and of high signal intensity foci on fat-saturated T1WI. Calcification-induced signal voids on SWAN were excluded by referring computed tomography (CT) images, or phase images obtained with SWAN. The reviewers examined all images of the cases independently and then resolved discrepancies by consensus. Twenty-two pathologically proven benign leiomyomas in seven women were also evaluated.Results and discussions
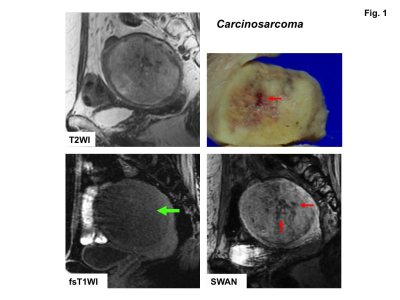
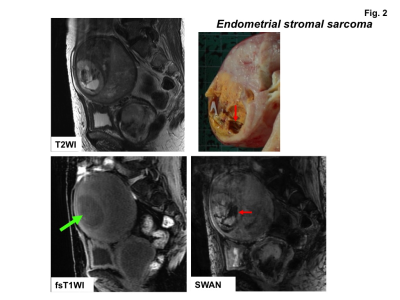
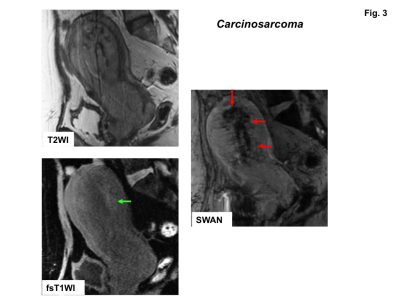
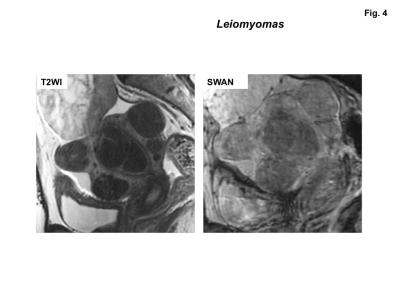
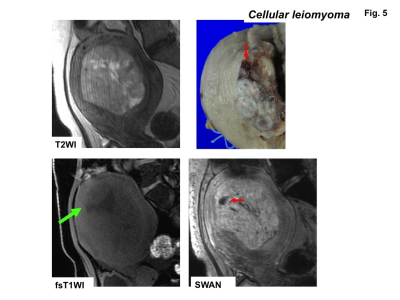
High signal intensity foci were detected in four of ten sarcomas (40%) on fat-saturated T1WI, whereas signal voids were detected in all sarcomas on SWAN (Fig.1-3). No high signal intensity foci on fat-saturated T1WI were observed in all 22 leiomyomas, and signal voids on SWAN were observed in only one leiomyoma (cellular leiomyoma) (Fig.4-5).
Hemorrhagic necrosis is often observed in sarcomas with coagulative necrosis due to the breakdown of tumor vasculature. The presence of high intensity areas on T1WI may be characteristic for intra-tumoral hemorrhage, although the prevalence is vary (18 to 63%)1-4). In our study only 40% of sarcomas showed high intensity on fat-saturated T1WI, whereas all lesions revealed signal voids on SWAN. That may be because high signal intensity due to the T1 shortening effect of methemoglobin may reflect only subacute hemorrhage, whereas signal voids on SWAN may reflect all phases of hemorrhage, especially both deoxyhemoglobin in acute phase and hemosiderin in chronic to obsolete phase.
Sehgal reported that SWI visualized blood products in high grade brain tumors and improved tumor characterization than T1WI. Because high-grade, aggressive tumors tend to have rapidly growing vasculature and multiple microhemorrhage or hemorrhagic necrosis may occur, detecting hemorrhagic areas within tumors could lead to improved determination of tumor status7-8). Takeuchi reported high intensity foci were detected in 50% of extra-ovarian endometriosis on fat-saturated T1WI, whereas signal voids were detected in all lesions (100%) on SWI, and concluded that SWI may improve the diagnostic ability of extra-ovarian endometriosis by demonstrating hemorrhage of varying chronicity9). In the current study, SWAN is sensitive for intra-tumoral hemorrhage in patients with sarcomas, and considered as useful sequence for the diagnosis of uterine sarcoma as high-grade malignant tumor.
Leiomyomas are the most common benign uterine neoplasm and are composed of smooth muscle and fibrous connective tissue. Leiomyomas may outgrow their blood supply as they enlarge, resulting in various types of degeneration10). However, hemorrhage and necrosis are not common (other than red degeneration) in benign leiomyomas11). In the current study, only one of 22 benign leiomyomas showed speckled intra-tumoral hemorrhage on SWAN. The leiomyoma with hemorrhage was histologically diagnosed as cellular leiomyoma. The other 21 of 22 usual leiomyomas with or without degeneration did not show intra-tumoral hemorrhage both on T1WI or on SWAN.
Conclusions
SW sequences such as SWAN are sensitive MR technique which demonstrate intra-tumoral hemorrhage of uterine sarcomas, and we conclude that the demonstration of intra-tumoral hemorrhage in patients suspected with uterine sarcomas by SW sequences may provide valuable diagnostic findings.Acknowledgements
No acknowledgement found.References
1) Santos P, Cunha TM. Uterine sarcomas: clinical presentation and MRI features. Diagn Interv Radiol 2015;21:4-9.
2) Sahdev A, Sohaib SA, Jacobs I, et al. MR imaging of uterine sarcomas. AJR 2001;177:1307-1311.
3) Tanaka YO, Nishida M, Tsunoda H, et al. Smooth muscle tumors of uncertain malignant potential and leiomyosarcomas of the uterus: MR findings. J Magn Reson Imaging 2004;20:998-1007.
4) Tanaka YO, Tsunoda H, Minami R, et al. Carcinosarcoma of the uterus: MR findings. J Magn Reson Imaging. 2008;28:434-439.
5) Haacke EM, Xu Y, Cheng YC, et al. Susceptibility weighted imaging (SWI). Magn Reson Med 2004;52:612-618.
6) Boeckh-Behrens T, Lutz J, Lummel N et al. Susceptibility-weighted angiography (SWAN) of cerebral veins and arteries compared to TOF-MRA. Eur J Radiol 2012;81:1238-1245.
7) Sehgal V, Delproposto Z, Haddar D, et al. Susceptibility-weighted imaging to visualize blood products and improve tumor contrast in the study of brain masses. J Magn Reson Imaging 2006;24:41-51.
8) Löbel U, Sedlacik J, Sabin ND, et al. Three-dimensional susceptibility-weighted imaging and two-dimensional T2*-weighted gradient-echo imaging of intratumoral hemorrhages in pediatric diffuse intrinsic pontine glioma. Neuroradiology 2010; 52:1167-1177.
9) Takeuchi M, Matsuzaki K, Harada M. Susceptibility-weighted MRI of extra-ovarian endometriosis: preliminary results. Abdom Imaging 2015;40:2512-2516.
10) Murase E, Siegelman ES, Outwater EK, et al. Uterine leiomyomas: histopathologic features, MR imaging findings, differential diagnosis, and treatment. Radiographics. 1999;19:1179-97.
11) Ueda H, Togashi K, Konishi I, et al. Unusual appearances of uterine leiomyomas: MR imaging findings and their histopathologic backgrounds. Radiographics. 1999;19 Spec No:S131-45.
Figures