4674
Reorganization of Homotopic Functional Connectivity in two Profiles of High Potential Children: a resting-state fMRI and DTI Study1CREATIS, Université Claude Bernard Lyon1, Lyon, France, 2Grenoble Institut des Neurosciences (GIN), Université Grenoble Alpe, Grenoble, France, 3GIPSA-lab, Université Grenoble Alpe, Grenoble, France, 4Service de Psychopathologie du Développement, Hopital Femme Mère Enfant, Hospices Civils de Lyon, Bron, France, 5Service de psychopathologie du développement de l'enfant et de l'adolescent, Hôpital Neurologique, Hospices Civils de Lyon, Bron, France, 6Laboratoire Parcours Santé Systémique (P2S), Université de Lyon, Lyon, France, 7Centre PSYRENE, Lyon, France, 8CERMEP - Imagerie du Vivant, Lyon, France
Synopsis
Several changes were previously found in functional and structural networks of High Potential (HP) children but interhemispheric communication was not investigated. In this study, we studied the homotopic connectivity between pairs of left and right hemispheres regions in three groups of children with standard IQ, homogeneous and heterogeneous high IQ. Functional and structural connectivity were investigated using resting-state fMRI and DTI tractography, respectively, and graph theory for brain network analysis. Our findings showed a reorganization of functional homotopic connectivity involving precuneus, amygdala and frontal pole regions in HP children.
Introduction
Along brain maturation, homotopic brain regions strengthen their connectivity with advancing age1 while the axonal organization and myelination processes occur leading to main fiber tracts development2. Importantly, the functional connections between homotopic areas are the most stable and less likely to change compared to any other type of connection3, allowing to study brain homotopy in normal and pathological conditions4. In particular, the cognitive functions related to intelligence, and quantified by the IQ tests, seemed to impact the functional connectivity5. In this work, we investigated brain connectivity in a population of high IQ children, as measured by the Wechsler Intelligence Scale for Children (WISC-IV), separated in two profiles based on their IQ subscores and corresponding to clinical observations. Indeed, the homogeneous HP profile (Hom-HP) usually shows well-controlled behavior and successful curriculum while the heterogeneous HP profile (Het-HP) often presents social maladjustment and learning troubles. Such psychological profile can be detected by a significant difference between verbal comprehension index (VCI) and perceptual reasoning index (PRI) values as well as standard levels in working memory index (WMI) and processing speed index (PSI). In a previous DTI-TBSS study, we have found that axial diffusivity was increased in the main white matter fiber-bundles, in particular in the corpus callosum, of both HP profiles compared to controls. The two HP profiles also showed hemispheric differences6. Therefore, we aimed in this work to investigate specifically their left-right communication using homotopic functional (FC) and structural connectivity (SC).Methods
Forty-four HP children (24 Het-HP and 20 Hom-HP) and 14 healthy control (HC) subjects with age mean of 10.1 (± 1.2) years-old were scanned with a 1.5T Siemens Sonata MRI system. MRI protocol consisted in a millimetric T1-MPR sequence, a resting-state fMRI BOLD-weighted EPI sequence (250 scans, TE/TR=50/2500ms, resolution=3.4x3.4x3mm) and a 2D-EPI DTI sequence (TE/TR=6900/86ms, resolution=2.5x2.5x2.5mm).
The rs-fMRI data were preprocessed using SPM12 software and data artifacts for head motion were detected using Art Toolbox. The scans affected by head motion bigger than 3 mm translation were removed. Subjects with more than 20% of removed scans were excluded at this stage. Time series were extracted using Conn Toolbox from a total of 84 regions, encompassing brain cortical and sub-cortical areas of Desikan Atlas. FC matrices were computed using the Brainwaver wavelet method7.
Diffusion data were corrected for subjects’ motion and Eddy currents, and non-brain voxels were removed using FSL. The tensor model was then fitted on diffusion data and the diffusion maps were extracted. Each subject FA map was co-registered to the one of the IIT atlas, using the affine and non-rigid algorithms of NiftyReg. The Desikan grey matter parcellation in the IIT atlas space was then used to define the 84 nodes of the graph. Based on the four-tissue-class classification of the IIT atlas, anatomically-constrained probabilistic streamline tractography was performed to generate 1000000 streamlines using orientation distribution function. Finally, adjacency matrices were generated for each subject summing the number of streamline connecting each pair of nodes.
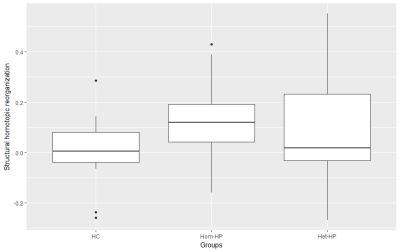
Connectivity between homotopic regions was directly derived from the connectivity matrix for statistical analysis. As proposed by Achard et al.8, the 'hub disruption index' κ was computed to assess a potential reorganization of homotopic FC and SC. κ differences between HP and HC groups were evaluated using ANOVA while regressing for gender.
Results
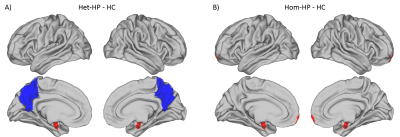
Boxplots of κ values measured in each group (fig.1&2) evidence a global reorganization of homotopic FC. In the Het-HP group, the homotopic FC was significantly decreased in the precuneus/posterior cingulate cortex (PCC) region, and increased in the amygdala region (fig.3A). In the Hom-HP group, the homotopic FC was also increased in amygdala and frontal pole regions (fig.3B). In contrast, the homotopic SC did not show any significant changes in white matter reorganization (fig.4).Discussion and Conclusion
These results highlighted an increased FC between homotopic amygdala regions in both HP groups that could be related to their high emotional sensibility. The Hom-HP group showed an increased homotopic FC in frontal pole, suggesting a strong ability for temporal planning and control of environmental stimulations. In contrast, the Het-HP group showed a decreased homotopic FC in precuneus/PCC, a set of regions involved in the perception of one's self. If the Het-HP children are believed to be self-oriented, this decrease in homotopic FC may confirm the hemispheric differences previously found in SC with left hemisphere dominance. The lack of significant changes in SC is probably explained by the small number of structural connections found between homotopic regions.
In conclusion, this study evidenced homotopic FC modifications in HP children that are supported by previous structural and functional measurements.
Acknowledgements
This work was performed within the framework of the LABEX PRIMES (ANR-11-LABX-0063) of Université de Lyon, within the program "Investissements d'Avenir" (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR) and it was made possible by APICIL Foundation.
References
1. Thomason, M. E. et al. Cross-Hemispheric Functional Connectivity in the Human Fetal Brain. Sci. Transl. Med. 5, 173ra24 (2013).
2. Zanin, E. et al. White matter maturation of normal human fetal brain. An in vivo diffusion tensor tractography study. Brain Behav. 1, 95–108 (2011).
3. Mišić, B. et al. The functional connectivity landscape of the human brain. PLoS One. 9, e111007 (2014).
4. Zuo, X.-N. et al. Growing Together and Growing Apart: Regional and Sex Differences in the Lifespan Developmental Trajectories of Functional Homotopy. J. Neurosci. 30, 15034–15043 (2010).
5. Santarnecchi, E. et al. Intelligence-related differences in the asymmetry of spontaneous cerebral activity. Hum. Brain Mapp. 36, 3586–3602 (2015).
6. Nusbaum, F. et al. Hemispheric Differences in White Matter Microstructure between Two Profiles of Children with High Intelligence Quotient vs. Controls: A Tract-Based Spatial Statistics Study. Front. Neurosci. 11, 1–11 (2017).
7. Achard, S. & Bullmore, E. Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 3, 0174–0183 (2007).
8. Achard, S. et al. Hubs of brain functional networks are radically reorganized in comatose patients. Proc. Natl. Acad. Sci. 109, 20608–20613 (2012).
Figures
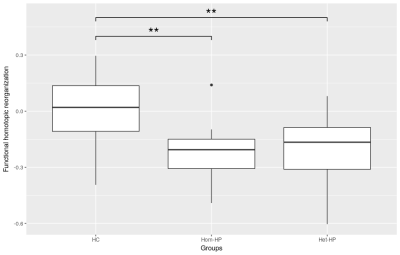
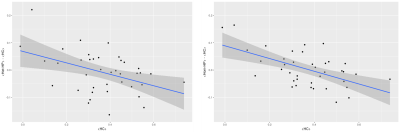
Fig 2: κ for functional homotopic connectivity of both HP groups comparing to Control group. The difference of homotopic FC means between HP group and HC group is plotted versus the homotopic FC mean of each node in the HC subjects. The slope of the blue line fitted to the data is the 'hub disruption index' κ. On the left for Het-HP and on the right for Hom-HP.