4673
COMT Val158Met polymorphism modulated the relationship between functional connectivity in prefrontal cortex and working memory performance1Department of Radiology, Drum Tower Hospital, Medical School of Nanjing University, Nanjing, China
Synopsis
Compared with participants with COMT rs4680-GG, the healthy Han Chinese participants with rs4680-A-allele showed higher degree centrality and functional connectivity in medial prefrontal cortex in resting-state BOLD. The rs4680-A-allele carriers also performed better in 3-back performance than rs4680-GG carriers, and the accuracy of 3-back has significant correlation with functional connectivity value, which indicated that the COMT rs4680 mutation modulated the functional connectivity and working memory performance.
Introduction
The catechol-O-methyltransferase (COMT) gene, which encodes COMT protein, is essentially involved in human emotion, reward processing, executive functions, and especially working memory. Among those single nucleotide polymorphism (SNP) loci[1] of COMT, Val158Met polymorphism was believed to play critical roles in human cognition[2, 3], which contains a G to A missense variant[4]. To further investigate how COMT gene affects the human brain in vivo, many studies have focused on task functional magnetic resonance imaging (fMRI)[5-9]. However, few studies have connected the rs-fMRI signals with working memory performance, and to our knowledge, using the region of interest (ROI) extracted from degree centrality (DC) map as the candidate hub of functional connectivity (FC) analyzing in this topic, has not been studied so far. We aimed to investigate the relationship between resting-state functional magnetic resonance imaging (rs-fMRI) signals in brain and working memory performance in COMT Val158Met polymorphism carriers.Methods
This study was approved by the local medical research ethics committee. Written informed consent was obtained. Eighty participants were included and divided into two groups based on genotype (28 participants with rs4680-A-allele, age 22.14±1.30 years, 14 Male/14 Female; 52 participants with rs4680-GG, age 22.82±1.77 years, 16 Male/36 Female). All participants underwent rs-fMRI and a battery of neuropsychological test, including 1-back and 3-back, which focus on the working memory performance. DC from rs-fMRI was compared between rs4680-A-allele and rs4680-GG genotype carriers. Then the ROI extracted from the significantly different region in DC map was used as the seed of the following FC analysis. The scores of neuropsychological test was analyzed by two-sample T-test, and spearman correlation analysis was proceeded between the scores and FC value in whole subjects and within different genotype groups.Results
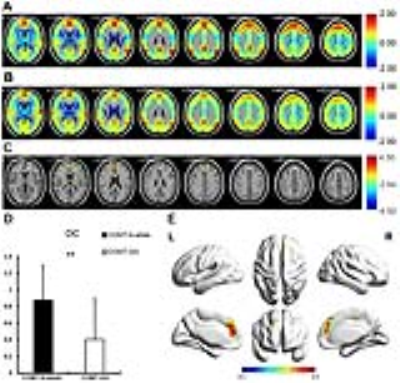
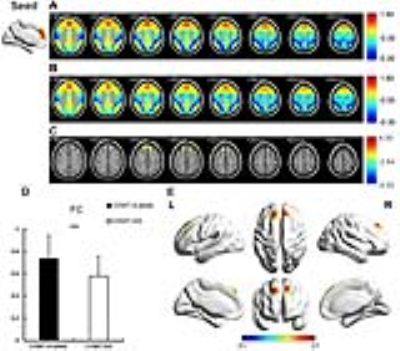
Compared with rs4680-GG carriers, rs4680-A-allele carriers showed significantly increased DC in bilateral superior medial frontal and bilateral anterior cingulum (Fig.1, p<0.01, AlphaSim corrected). Using the ROI extracted from the significantly different regioin in DC map as seeds, the FC value increased significantly in left superior frontal, right superior medial frontal, right superior frontal and left superior medial frontal in rs4680-A-allele group (Fig.2, p<0.01, AlphaSim corrected). The A-allele group had better performance in some variables of California verbal learning test (p<0.05) and 3-back accuracy (p=0.04). Spearman correlation between FC value and neuropsychological tests in all subjects was proceeded but did not reach significantly different level. However, significant negative correlation was showed between FC value and 3-back accuracy in rs4680-A-allele group, while rs4680-GG group did not reach statistical difference. The same trend did show in the within-group correlation between 1-back accuracy and FC value. The 1-back and 3-back accuracy had significant difference inside genotype groups, and the difference between 3-back and 1-back accuracy showed statistical difference in the correlation with FC value within A-allele group. However, the tendency between all the above-mentioned within-group correlation did not show obvious difference (Fig.3).Discussion
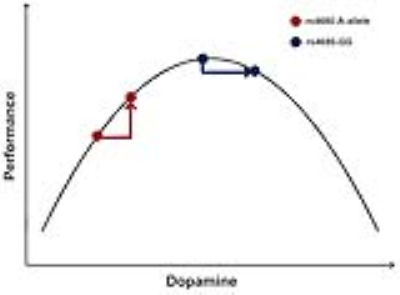
Previous studies have indicated that humans with Met allele have increased cortical dopamine signaling[7] due to the different enzymatic activity compared with Val allele[2, 3, 10], which probably indicates higher PFC activation and better performance during working memory[9, 11, 12]. Some researchers have found that increased PFC signals and better working memory associated with the A-allele participants[13-16], while others found opposite conclusion[6, 8]. This suggests a more complicated mechanism might be necessary to explain the effect of COMT on PFC. Many researches have implied that the inverted U model could explain the dopaminergic regulation of prefrontal function[10, 17]. It assumed that both insufficient and excessive dopamine level would lead to poorer performance. Inside the A-allele group with more dopamine in PFC, the higher FC level they had, the lower accuracy they performed in 3-back, which indicated the increased difficulty of task needs more brain activity. And the GG-group had the opposite tendency, although it did not reach statistical significant level, which both accorded with the inverted U model (Fig.4).Conclusion
Our results suggest that the rs4680-A-allele genotype group shows significantly higher DC and FC in prefrontal cortex, and better working memory performance compared with rs4680-GG group. The 1-back and 3-back accuracy had significant difference inside genotype groups, while significantly negative correlation was showed between FC value and 3-back accuracy in rs4680-A-allele group, which is the same trend between FC and the difference between 3-back and 1-back. These findings advance our understanding of the relationship among COMT genotype, intrinsic activity in prefrontal cortex and the performance in working memory, which may be a baseline for future researches about how the dopamine affects spontaneous fluctuation in rs-fMRI and the human cognition.Acknowledgements
This work was supported by the National Natural Science Foundation of China (81720108022, 91649116, 81571040, B.Zhang, 81471643, B.Zhu.); key medical talents of the Jiangsu province, the "13th Five-Year" health promotion project of the Jiangsu province (B.Z.2016-2020); the social development project of science and technology project in Jiangsu Province (BE2016605, BE201707); Jiangsu Provincial Key Medical Discipline (Laboratory) (ZDXKA2016020); the National and Provincial postdoctoral project (BE179 and 1501076A, BZ); the key project of Nanjing Health Bureau (ZKX14027, BZ); the Nanjing science and technology development program (NE179, B.Z.); and the project of the sixth peak of talented people (WSN-O50, BZ). National Key R&D Program of China (2016YFC0100100). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.References
1. Chen, X., et al., Variants in the catechol-o-methyltransferase (COMT) gene are associated with schizophrenia in Irish high-density families. Molecular psychiatry, 2004. 9(10): p. 962.
2. Bertolino, A., et al., Interaction of COMT Val108/158 Met genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. American Journal of Psychiatry, 2004. 161(10): p. 1798-1805.
3. Blasi, G., et al., Effect of catechol-O-methyltransferase val158met genotype on attentional control. Journal of Neuroscience, 2005. 25(20): p. 5038-5045.
4. Lachman, H.M., et al., Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics and Genomics, 1996. 6(3): p. 243-250.
5. Green, A.E., et al., A gene–brain–cognition pathway: prefrontal activity mediates the effect of COMT on cognitive control and IQ. Cerebral cortex, 2012. 23(3): p. 552-559.
6. Bertolino, A., et al., Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. Journal of Neuroscience, 2006. 26(15): p. 3918-3922.
7. Egan, M.F., et al., Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences, 2001. 98(12): p. 6917-6922.
8. Tan, H.-Y., et al., Epistasis between catechol-O-methyltransferase and type II metabotropic glutamate receptor 3 genes on working memory brain function. Proceedings of the National Academy of Sciences, 2007. 104(30): p. 12536-12541.
9. Caldú, X., et al., Impact of the COMT Val 108/158 Met and DAT genotypes on prefrontal function in healthy subjects. Neuroimage, 2007. 37(4): p. 1437-1444.
10. Mattay, V.S., et al., Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proceedings of the National Academy of Sciences, 2003. 100(10): p. 6186-6191.
11. Mier, D., P. Kirsch, and A. Meyer-Lindenberg, Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Molecular psychiatry, 2010. 15(9): p. 918.
12. Bertolino, A., et al., Prefrontal dysfunction in schizophrenia controlling for COMT Val 158 Met genotype and working memory performance. Psychiatry Research: Neuroimaging, 2006. 147(2): p. 221-226.
13. Congdon, E., et al., Influence of SLC6A3 and COMT variation on neural activation during response inhibition. Biological psychology, 2009. 81(3): p. 144-152.
14. Winterer, G., et al., COMT genotype predicts BOLD signal and noise characteristics in prefrontal circuits. Neuroimage, 2006. 32(4): p. 1722-1732.
15. Bertolino, A., et al., Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biological psychiatry, 2006. 60(11): p. 1250-1258.
16. Winterer, G. and D.R. Weinberger, Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends in neurosciences, 2004. 27(11): p. 683-690.
17. Williams, G. and S. Castner, Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience, 2006. 139(1): p. 263-276.
Figures