4665
Alteration of resting state functional connectivity following cocaine self-administrationSung-Ho Lee1,2, Heather K Decot1,2,3,4, Fei Fei Wang5, Regina M. Carelli5,6, Yen-Yu Ian Shih1,2,3,6,7,8, and Garret D Stuber3,4,6,8
1Neurology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 2Biomedical Research Imaging Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 3Curriculum in Neuroscience, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 4Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 5Psychology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 6Neuroscience Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 7Biomedical Engineering, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 8Cell Biology and Physiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
Synopsis
We investigate the alteration of resting-state functional connectivity across the brain following self-administration of cocaine in the rat. The result of group-independent component analysis (ICA) and dual regression reveals that cocaine self-administration orchestrates dynamic shifts in co-activity and functional connectivity across resting-state neuronal networks and nodes, several of which that do not directly receive dopamine input.
Introduction
Cocaine is a powerfully addictive stimulant drug that exerts its reinforcing effects, at least in part, through the mesolimbic dopamine (DA) system1-3. While our understanding of the molecular, cellular and structural drug-induced changes following repeated cocaine exposure has advanced4-7, much less is known about the circuit-level neuroadaptations that occur. Functional connectivity magnetic resonance imaging (fcMRI) has emerged as a powerful tool to investigate large-scale brain network and provides information about neuronal connectivity across the brain8-10. Previous human fcMRI have revealed that cocaine dependence is associated with alterations of connectivity of brain networks11-13. However, confounding factors inherent to human subject research make it difficult to isolate the direct impact of cocaine exposure on the brain14,15. Here, we used a pre-post, treatment-control design, which allowed us to measure brain-wide functional connectivity before and after cocaine exposure in the same animal and to compare these findings to a control group using group-ICA16 and dual regression approach17. This study provides critical insight into the circuit-level maladaptations that underlie the escalation from voluntary use of the drug to the compulsive drug-seeking behavior.Methods
Adult male Sprague Dawley rats (initially ~300 g from Charles River Laboratories) were used. These subjects were mildly water restricted to ~20 ml/d during the self-administration portion of the experiment. All procedures are approved by the Institutional Animal Care and Use Committee at the project institution. The intrajugular catheters are surgically implanted to rats, and intravenous cocaine (n = 7, 1.67 mg/ml in 0.9% saline) or saline (n = 6, 0.9% saline) are randomly assigned to two self-administration groups (Figure 1). During the fMRI acquisition, each rat was endotracheally intubated for ventilation. The vital signs including body temperature (37 ± 0.5ºC), end-tidal CO2 (3.0±0.2%), heart rate (280 ± 20 bpm) and oxygen saturation (>96%) were continuously monitored and maintained. Continuous infusion of dexmedetomidine (0.05 mg/kg/hr) and pancuronium bromide (0.5 mg/kg/hr) with 0.5% isoflurane are used to maintain stable sedation. We performed two scanning sessions – one before surgery, and another after 10 days of self-administration. Each scanning session contains 6 consecutive 5-min fcMRI scans. All fMRI data were acquired using a 9.4 Tesla Bruker BioSpec MRI scanner with a 72 mm volume transmitter, and a 4-channel phased array receiver. BOLD fcMRI scans were acquired using a single shot isotropic gradient echo-EPI sequence (spectral width=150 kHz, TR/TE=2000/11.2 ms, FOV=2.88x2.88x1.28 cm, slices = 32, matrix=72x72 and spatial resolution=0.4 mm isotropic). For data preprocessing, we used standard pipelines18,19 including slice timing correction, motion correction, spatial normalization, and smoothing (FWHM=0.5mm). The six motion parameters and high-pass filter (>0.01 Hz) were applied for the temporal filtering of the data. For multiple comparison correction, we used family-wise error rate (FWER) with autocorrelation correction to estimate the cluster size at p-value = 0.01 and α = 0.0520,21. AFNI, ANTs, FSL, and python scikit-learn module were used for preprocessing, data analysis and visualization.Results
The group-ICA were performed (n=30, d=30) to derive resting-state networks (RSNs) from the baseline scans acquired from all subjects (n=13). Following inspection of the 30 components, 11 of them were classified as noise components. The remaining 19 components (Figure 2) were each classified as an RSN, consistent with the findings reported previously9,10,22-28. We perform dual regression to investigate whether correlated activity between RSN and specific brain node was altered following cocaine exposure. Significant increases of coactivity were observed between the anterior cingulate cortex (ACC) and the visual network (ΔZ = -1.94 ± 0.43; Figure 3A) and between the somatosensory cortex and the sensorimotor network (ΔZ = -2.31 ± 0.43; Figure 3B). No significant difference was observed in the control group. Finally, seed-based connectivity analysis was performed to further investigate whether these regions exhibit alteration in connectivity strength with other brain structures. We found that ACC seeds had a significant reduction in functional connectivity with the dorsal striatum (ΔZ = 0.135 ± 0.03; Figure 4). No significant difference was observed in other seed regions or the control group. differences were observed in the control animals.Discussions
Our findings demonstrate that cocaine exposure can cause significant global network remodeling in the drug naïve brain, with ACC being one of the major region that involve these alteration (Figure 5). These findings may reflect key network-level alterations between reward and sensory-related brain circuits, and underlying the maladaptive behavioral outcomes seen in cocaine dependence. Importantly, this project provides a means to identify novel neuroanatomical circuit nodes associated with phases of addiction, and for future experiments to explore whether network-based interventions can normalize maladaptive network activity.Acknowledgements
We thank members of the Shih and Stuber labs for valuable discussions concerning the studies described in this abstract. Our team is supported by NIMH R01MH111429, R41MH113252, R21 MH106939, NINDS R01NS091236, NIAAA U01AA020023, NIDA R01 DA032750, R01 DA038168, F31 041104, T32 NS007431, R01AA025582, NICHD U54HD079124, UNC Graduate Training Program in Translational Medicine supported by HHMI, American Heart Association 15SDG23260025, The Brain & Behavior Research Foundation. The Foundation of Hope, and The Klarman Family Foundation.References
- Aragona, B. J., Cleaveland, N. A., Stuber, G. D., Day, J. J., Carelli, R. M., & Wightman, R. M. (2008). Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(35), 8821–8831.
- https://doi.org/10.1523/JNEUROSCI.2225-08.2008 Di Chiara, G., & Imperato, A. (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America, 85(14), 5274–5278.
- Stuber, G. D., Roitman, M. F., Phillips, P. E. M., Carelli, R. M., & Wightman, R. M. (2005). Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 30(5), 853–863. https://doi.org/10.1038/sj.npp.1300619
- Conrad, K. L., Tseng, K. Y., Uejima, J. L., Reimers, J. M., Heng, L.-J., Shaham, Y., … Wolf, M. E. (2008). Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature, 454(7200), 118–121. https://doi.org/10.1038/nature06995
- Muñoz-Cuevas, F. J., Athilingam, J., Piscopo, D., & Wilbrecht, L. (2013). Cocaine-induced structural plasticity in frontal cortex correlates with conditioned place preference. Nature Neuroscience, 16(10), 1367–1369. https://doi.org/10.1038/nn.3498
- Stuber, G. D., Hopf, F. W., Tye, K. M., Chen, B. T., & Bonci, A. (2010). Neuroplastic alterations in the limbic system following cocaine or alcohol exposure. Current Topics in Behavioral Neurosciences, 3, 3–27. https://doi.org/10.1007/7854_2009_23
- Ungless, M. A., Whistler, J. L., Malenka, R. C., & Bonci, A. (2001). Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature, 411(6837), 583–587. https://doi.org/10.1038/35079077
- Biswal, B., Yetkin, F. Z., Haughton, V. M., & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541.
- Hutchison, R. M., Mirsattari, S. M., Jones, C. K., Gati, J. S., & Leung, L. S. (2010). Functional networks in the anesthetized rat brain revealed by independent component analysis of resting-state FMRI. Journal of Neurophysiology, 103(6), 3398–3406. https://doi.org/10.1152/jn.00141.2010
- Lu, H., Zou, Q., Gu, H., Raichle, M. E., Stein, E. A., & Yang, Y. (2012). Rat brains also have a default mode network. Proceedings of the National Academy of Sciences of the United States of America, 109(10), 3979–3984. https://doi.org/10.1073/pnas.1200506109
- Gu, H., Salmeron, B. J., Ross, T. J., Geng, X., Zhan, W., Stein, E. A., & Yang, Y. (2010). Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. NeuroImage, 53(2), 593–601. https://doi.org/10.1016/j.neuroimage.2010.06.066
- Tomasi, D., Volkow, N. D., Wang, R., Carrillo, J. H., Maloney, T., Alia-Klein, N., … Goldstein, R. Z. (2010). Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PloS One, 5(5), e10815. https://doi.org/10.1371/journal.pone.0010815
- Wilcox, C. E., Teshiba, T. M., Merideth, F., Ling, J., & Mayer, A. R. (2011). Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug and Alcohol Dependence, 115(1–2), 137–144. https://doi.org/10.1016/j.drugalcdep.2011.01.009
- Compton, W. M., Thomas, Y. F., Stinson, F. S., & Grant, B. F. (2007). Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry, 64(5), 566–576. https://doi.org/10.1001/archpsyc.64.5.566
- Greenland, S., Pearl, J., & Robins, J. M. (1999). Causal diagrams for epidemiologic research. Epidemiology (Cambridge, Mass.), 10(1), 37–48.
- Beckmann, C. F., DeLuca, M., Devlin, J. T., & Smith, S. M. (2005). Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 360(1457), 1001–1013. https://doi.org/10.1098/rstb.2005.1634
- Beckmann, C. F., Mackay, C. E., Filippini, N., Smith, S.M., (2009). Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. NeuroImage, 47(s1), S148. https://doi.org/10.1016/S1053-8119(09)71511-3
- Broadwater M. A, Lee, S. H., Yu, Z. H., Crews, F. T., Robinson, D. L., Shih, Y. Y. (2017). Addiction Biology, In Press. https://doi.org/10.1111/adb.12530 2.
- Decot, H., Namboodiri, V. M. K., Gao, W., McHenry, J., Jennings, J., Lee, S. H., Kantak, P., Kao, Y. C., Das, M., Witten, I., Disseroth, K., Shih, Y. Y., Stuber, G. D. (2016) Neuropsychopharmacology, 42 (3): 615-627.
- Cox, R. W., Chen, G., Glen, D. R., Reynolds, R. C., & Taylor, P. A. (2017). FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connectivity, 7(3), 152–171. https://doi.org/10.1089/brain.2016.0475
- Eklund, A., Nichols, T. E., & Knutsson, H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 7900–7905. https://doi.org/10.1073/pnas.1602413113
- Becerra, L., Pendse, G., Chang, P.-C., Bishop, J., & Borsook, D. (2011). Robust reproducible resting state networks in the awake rodent brain. PloS One, 6(10), e25701. https://doi.org/10.1371/journal.pone.0025701
- Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. https://doi.org/10.1196/annals.1440.011
- Fox, M. D., Raichle, M. E, (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience 8, 700-711. https://doi.org/10.1038/nrn2201
- Ma, Z., Perez, P., Ma, Z., Liu, Y., Hamilton, C., Liang, Z., Zhang, N. (2016). Functional atlas of the awake rat brain: A neuroimaging study of rat brain specialization and integration. NeuroImage, In Press, https://doi.org/10.1016/j.neuroimage.2016.07.007
- Medda, A., Hoffmann, L., Magnuson, M., Thompson, G., Pan, W., Keilholz, S. (2017). Wavelet-based clustering of resting state MRI data in the rat. Magn Reson Imaging. 34(1): 35-43. https://doi.org/10.1016/j.mri.2015.10.005
- Chuang, K., Nasrallah, F. A., (2017). Functional networks and network pertubations in rodents. NeuroImage. In Press, https://doi.org/10.1016/j.neuroimage.2017.09.038
-
Gozzi,
A., Schwarz, A. J., (2015). Large-scale functional connectivity networks in the
rodent brain. NeuroImage. 15(127): 496-509. https://doi.org/10.1016/j.neuroimage.2015.12.017
Figures
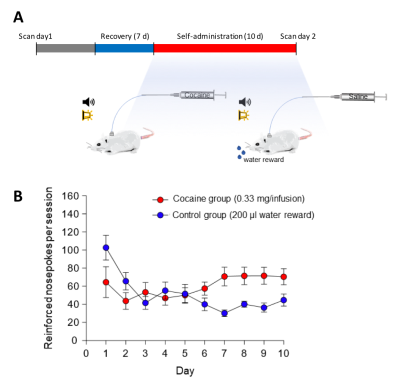
Figure 1. Experimental timing diagram and self-administration training data. (A) All animals underwent 6 consecutive 5-minute rsfMRI scans prior to self-administration, immediately following self-administration (within 24-48 hr following last session). Self-administration sessions were 6 h per day for 10 days. (B) Mean self-administration rates over the 10 days of training for rats nose-poking for cocaine (n =7) or for water (n =6). Error bars represent ±SEM.
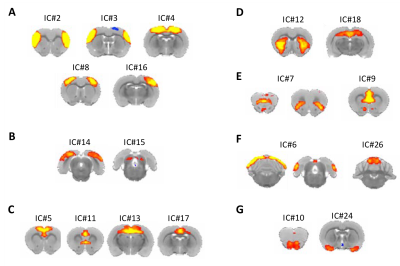
Figure 2. Resting state networks derived from the multi-subject ICA analysis. (A) Somatosensory (IC# 2,3) and sensorimotor networks (IC# 4, 8,16). (B) Visual networks (IC# 14,15). (C) Anterior default mode networks (IC# 5, 11) and posterior default mode networks (IC# 13, 17). (D) Striatal network (IC# 12) and hippocampal network (IC# 18). (E) Fronto-insular network (IC# 7) and thalamacortical network (IC# 9). (F) Cerebellar networks (IC# 6, 26). (G) Olfactory networks (IC# 10, 24).
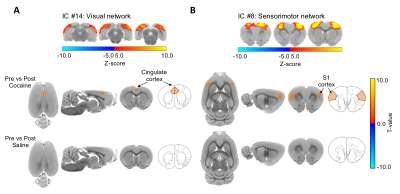
Figure 3.
Difference map of dual regression analysis between
pre-cocaine and post-cocaine rsfMRI. (A) Cocaine exposure causes a significant
increase in functional coactivity between the visual network and the anterior
cingulate cortex in drug naïve rats, (FWER-corrected, p-value = 0.01, ΔZ =
-1.94 ± 0.43). (B) Cocaine exposure causes a significant increase in functional
coactivity between the sensorimotor network and the primary somatosensory
cortex in drug naïve rats (FWER-corrected, p-value = 0.01, ΔZ = -2.31 ± 0.43). No differences between
condition were observed in the control group.
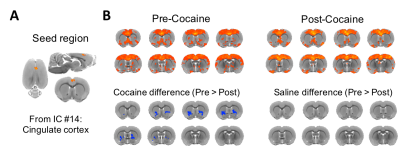
Figure 4. Seed-based brain connectivity maps of pre-cocaine and post-cocaine rsfMRI data using the (A) anterior cingulate cortex seed derived from the dual regression analysis. (B) Group analysis was performed to compare between group effects and revealed a significant reduction in functional connectivity between the anterior cingulate cortex and the striatum (NAc and ventral portion of CPu) following exposure to cocaine in drug naïve animals (FWER corrected, p-value = 0.01; ΔZ = 0.143 ± 0.03). No differences between condition were observed in the control group.
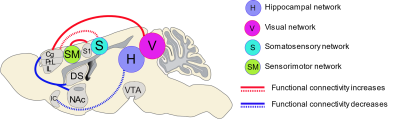
Figure
5.
Summary of functional connectivity changes following cocaine exposure in drug
naïve rats. Sagittal view of the rat brain showing the statistically
significant (FWER-corrected, p-value = 0.01) increases (red lines) and
decreases (blue lines) in functional connectivity across the brain following
cocaine exposure in drug naïve rats. The solid lines indicate the alteration of
co-activation between network and node, and dash lines indicate the alteration
of connectivity strength between brain regions.