4661
Resting state DfMRI revealed alterations of brain activity and network in obsessive–compulsive disorder mouse model1Departemnt of Neuropsychiatry, Keio University School of Medicine, Tokyo, Japan, 2ATR Brain Information Communication Research Laboratory Group, Kyoto, Japan, 3Neural Computation Unit and Biological Physics Theory unit, Okinawa Insititute of Science and Technology, Okinawa, Japan, 4Department of Molecular Neuroscience, Medical Research Institute, Tokyo Medical and Dental University, Tokyo, Japan
Synopsis
Diffusion fMRI (DfMRI) is a powerful functional imaging method to investigate neural activity and network without BOLD confounding hemodynamic effects. In this study, we applied DfMRI to evaluate brain activity and network of neuropsychiatric disease model, obsessive–compulsive disorder (OCD). Our DfMRI study revealed that the cortex was excited and the hippocampus was inhibited. Functional connectivity analysis of DfMRI detected alterations of cortico-striatal-thalamic networks, in line with the previous study with OCD patients. Additionally, we found that interesting network alterations of the hippocampal networks in this OCD model mice.
Purpose
Diffusion fMRI (DfMRI)1 has been shown to reflect neural activity, as DfMRI signals are not of vascular origin2, suggesting that DfMRI has an advantage to evaluate neural activity regardless of the status of hemodynamic responses. Previous study has suggested that water apparent diffusion coefficients (ADCs) obtained from the resting state DfMRI (rsDfMRI) closely reflected basal neural activity although hemodynamics was disrupted by anesthesia3. In addition, the study has proposed the method yielding DfMRI-guided cell swelling-composed networks3.
To show the applicability of rsDfMRI, we selected a well conceptualized neuropsychiatric animal model, an obsessive–compulsive disorder (OCD) model mouse, in which the cortico-striatal projection activated4 and the functional connectivity (FC) of cortico-striatal-thalamic networks altered5. There were two primary outcomes in this study. One was to detect the expected neural activity in the cortex. The other was to detect the expected alterations of cortico-striatal-thalamic networks. The secondary outcome was to find unexplored brain regions where previous BOLD-fMRI failed to extract.
Methods
We employed astrocytic glutamate transporter (GLT1) knockdown mice, which is known as OCD model6. The OCD model mouse was homozygous GLT1flox/flox with heterozygous inducible Cre recombinase (CreERT2) in GLAST locus (GLASTCreERT2/+) (GLASTCreERT2/+;GLT1 flox/flox, n=15) (Fig. 1A). GLAST+/+;GLT1flox/flox mice were used as the control mice (ctl, n=14). Both groups were intraperitoneally injected with 100 mg/kg tamoxifen (TAM) at postnatal days 17-21 for consecutive five days. We conducted a surgery to put an acclimation bar on the skull at 8 weeks of age, started the MRI acclimation7 at 10 weeks of age, and finished the acclimation at 12 weeks of age.
MRI were conducted under awake condition at 11.7T equipped with cryoprobe (BioSpec, Bruker). rsDfMRI images were acquired with the following parameters: diffusion-sensitized double SE-EPI sequence; TR/TE=2000/37 ms, FOV=16×16 mm2, Matrix=80×80, Slice thickness=0.8 mm. Slice number=10, Repetition number=150, Scan time=10 min, and b-values=1000 and 1800 mm2/s along 1 directions; [X=1,Y=1,Z=1]. Breathing and body temperature were monitored during scanning.
Pre-processing (realignment, slice timing, normalization) was performed using SPM12 and spatial smoothing was conducted with 0.4 mm of full width at half maximum. ADCs were calculated from time series data of diffusion weighted signal acquired with b=1000 and 1800 mm2/s (Sb1000, Sb1800) for each time point as ADC=ln(Sb1000/Sb1800)/800. Statistical analysis of ADCs was performed two-way repeated ANOVA over time and genotype.
Time-series data of Sb1800 were detrended and temporally filtered with 0.008-0.1 Hz. Seed-based FC analysis was performed using time-series data of pre-processed Sb1800. Regions of interest (ROIs) of 157 brain loci were defined with the Allen Mouse Brain Atlas. Pearson’s correlation coefficients between the time course of a predetermined ROI and all other ROIs were estimated after de-noising and de-spiking using functional connectivity toolbox (CONN) in order to generate a 157×157 connectivity matrix. Global mean signal was regressed out from the pre-processed time-series data in order to reduce non-neural signal correlations. Regions of white matter and cerebrospinal fluid were masked out.
Results and Discussion
GLT1 protein expression was significantly knocked down in the cortex (the cingulate cortex and the somatosensory cortex) and the hippocampus, confirming TAM-induced Cre mediated GLT1 deletion (Fig. 1B, C). ADCs were significantly decreased at the cortex in the OCD model compared with the ctl (Fig. 2), reflecting increased basal activity in the cortex in the OCD model. In FC analysis derived from rsDfMRI, we found altered cortico-striatal-thalamic functional connectivity in the OCD model (Fig. 3 and Table 1) as seen in the human studies from BOLD-fMRI5. These data demonstrated that rsDfMRI was able to detect expected alterations in OCD model mouse, showing the applicability of rsDfMRI.
We also found that ADC increased at the hippocampus (Fig. 2), reflecting decreased neural activity in the OCD model. This hippocampal inhibition was due to the decrease in hippocampal GLT1 level (Fig. 2) or due to the increase of an input from the entorhinal cortex (Table 2). FC of the hippocampal networks decreased in the OCD model (Fig. 5). The conventional BOLD-fMRI study has not reported this hippocampal alteration in the OCD patients. Our study proposed that the hippocampus was a new target region in OCD which was supported by a glutamate hypothesis.
Conclusion
Our rsDfMRI detected that cortical excitability and the altered FC of cortico-striatal-thalamic networks which agreed with the BOLD-fMRI study with the OCD patients, showing the applicability of rsDfMRI to a neuropsychiatric animal model. Interestingly, our rsDfMRI study indicated that the hippocampus had a possibility of a new target to understand a OCD mechanism.Acknowledgements
We thank Jiafu Zeng for animal support with mouse acclimation for MRI acquisition.References
1. Le Bihan D, Urayama S, Aso T et al. Direct and fast detection of neuronal activation in the human brain with diffusion MRI. Proc Natl Acad USA. 2006;103(21):8263-8268.
2. Tsurugizawa T, Ciobanu L, Le Bihan D. Water diffusion in brain cortex closely tracks underlying neuronal activity. Proc Natl Acad USA. 2013;110(28):11636-11641.
3. Abe Y, Tsurugizawa T, Le Bihan D. Water diffusion closely reveals neural activity status in rat brain loci affected by anesthesia. PloS Biol. 2017;15(4):e2001494.
4. Ahmari SE, Spellman T, Douglass NL et al. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340(6137):1234-1239.
5. Hou JM, Zhao M, Zhang W et al. Resting-state functional connectivity abnormalities in patients with obsessive-compulsive disorder and their healthy first-degree relatives. J Psychiatry Neurosci. 2014;39(5):304-311.
6. Aida T, Yoshida J, Nomura M et al. Astroglial glutamate transporter deficiency increases synaptic excitability and leads to pathological repetitive behaviors in mice. Neuropsychopharmacology. 2015;40(7): 1569-1579.
7. Yoshida K, Mimura Y, Ishihara R et al. Physiological effects of a habituation procedure for functional MRI in awake mice using a cryogenic radiofrequency probe. J Neurosci Methods. 2016;274:38-48.
Figures
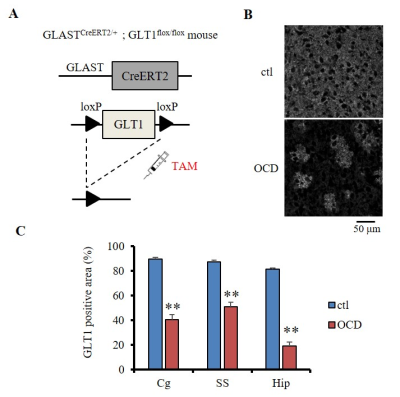
Fig. 1
(A) A loxP-flanked GLT1 exon 2 was excised by Cre recombinase, leading to GLT1 deletion. CreERT2, tamoxifen (TAM)-inducible Cre, was expressed only in astrocytes under the control of GLAST promoter. (B) Representative images of immunohistochemistry of GLT1 protein (white signal) at the cortex in ctl and OCD. The neuropil in the ctl cortex was filled with GLT1 immunoreactivity. GLT1 positive astrocytes were highlighted by surrounding GLT1 null astrocytes after recombination. (C) Percentage of GLT1 positive area at cingulate cortex (Cg), somatosensory cortex (SS), and the hippocampus (Hip). ** p<0.001 (t-test, v.s. the ctl of each region)
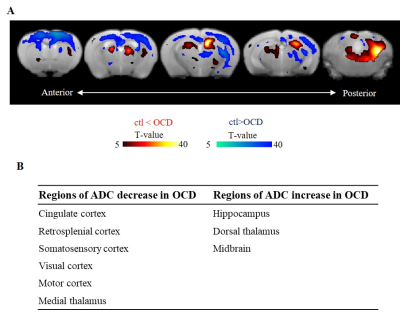
Fig. 2
(A) ADC magnitudes were compared between ctl and OCD mice (p<0.05, family wise error). Hot color means an ADC increase (neural deactivation) in OCD mice and cool color means an ADC decrease (neural activation). (B) The table represents the regions showing significant (p<0.05, t-test v.s. the ctl) decreases or increases in average ADCs in OCD mice, compared with ctl mice.
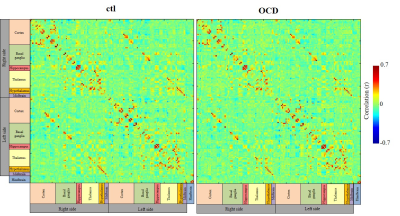
Fig. 3
Color matrices show correlation coefficients of each ROI in ctl and OCD mice. These ROIs were defined with the Allen Mouse Brain Atlas (both sides of 25 cortical areas, 18 basal ganglia, 4 hippocampal areas, 18 thalamic areas, 4 hypothalamic areas, and 3 midbrain areas and 13 hindbrain areas).
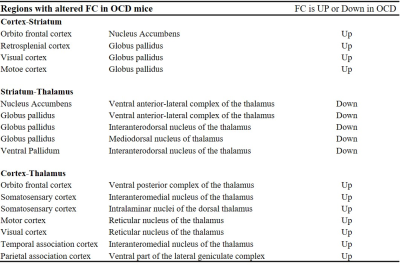
Table 1
The table shows three networks with significantly altered FC in OCD mice; cortex-striatum networks, striatum-thalamus networks, and cortex-thalamus networks.
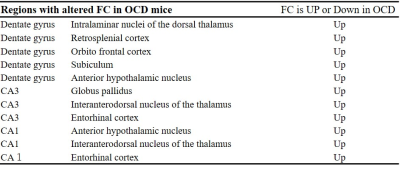
Table 2
The table shows the hippocampal networks with significantly altered FC in OCD mice.