4655
Serotonin transporter occupancy predicts default-mode network connectivity: a SPECT and rsfMRI study1Department of Radiology and Nuclear Medicine, Academic Medical Center, University of Amsterdam, Amsterdam, Netherlands, 2Spinoza Centre for Neuroimaging, Royal Netherlands Academy of Arts and Sciences (KNAW), Amsterdam, Netherlands, 3Swammerdam Institute for Life Sciences, Center for Neurosciences, University of Amsterdam, Amsterdam, Netherlands
Synopsis
The serotonergic neurotransmitter system is thought to play a substantial role in modulating the default mode network (DMN). For example, antidepressants (SSRIs) have consistently shown to decrease DMN connectivity. However, it is unclear whether SSRIs also dose-dependently affect DMN connectivity. Therefore, we investigated the association between SERT occupancy by SSRIs (SPECT) and DMN functional connectivity (rs-fMRI). We confirm a dose-dependent effect of SSRIs on connectivity with the DMN; higher SERT occupancy by the SSRI in the thalamus was significantly associated with decreased DMN connectivity. This suggests that DMN connectivity might be interesting biomarker, e.g. for treatment monitoring.
Introduction
The default-mode network (DMN) is characterized by being most active when a subject is not performing a particular task and can be assessed using resting-state functional magnetic resonance imaging (rs-fMRI). Several studies have suggested a substantial role for the serotonergic neurotransmitter system in modulating the DMN, as its receptor expression has considerable spatial overlap with the DMN regions. In addition, serotonin neurons in the medial and dorsal raphe nuclei innervate the DMN regions through efferent projections1. Selective serotonin reuptake inhibitors (SSRIs), typically used as antidepressants in the treatment of major depression, have also consistently shown to decrease DMN connectivity1. Molecular imaging studies have shown that around 80% occupancy of the serotonin transporter (SERT) is optimal to obtain therapeutic effects of SSRIs2. However, it is unclear whether SSRIs also dose-dependently affect DMN connectivity. Therefore, the focus of the current study was to investigate the association between SERT occupancy by SSRIs (assessed with SPECT) and functional connectivity with the DMN (assessed with rs-fMRI).Methods
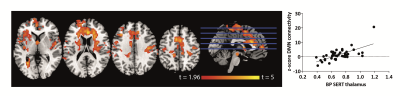
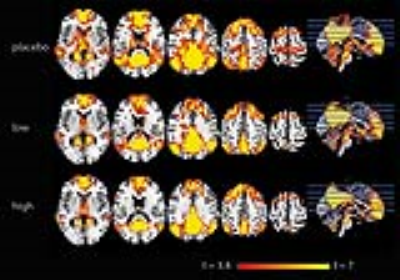
Forty-five healthy female volunteers (mean age=21.6y) participated in a double-dose study investigating SERT occupancy (SPECT) and DMN connectivity (rs-fMRI). The subjects received pre-treatment with placebo, low (4 mg; ‘low group’) or clinically standard (16 mg; ‘high group’) oral citalopram dose (corresponding to 0%, ~40% and ~80% SERT occupancy, respectively3). Subsequently, they underwent a SPECT scan with [123I]FP-CIT, which binds predominantly to the SERT in the thalamus4. After the SPECT scan, subjects underwent a rs-fMRI scan. Citalopram blood plasma levels were also measured. SPECT scans were acquired using an InSPira-HD SPECT camera (Neurologica) with the following parameters: matrix=121x121; slice thickness=4mm, acquisition time per slice=180s. They were reconstructed in 3D mode, attenuation-corrected and spatially smoothed (3mm). SPECT images were coregistered with the individual 3DT1-weighted (T1w) MR image using SPM. ROI analysis was performed to determine SERT binding in the thalamus. Thalamic masks were extracted from individual T1w scans using Freesurfer. The cerebellum was used as a reference region to assess non-specific binding. Specific to non-specific binding ratios (binding potential: BPND) were calculated as follows: (mean thalamic binding - mean cerebellum binding)/mean cerebellum binding. MRI data were acquired using a 3.0T Ingenia (Philips) equipped with a 32-channel receive-only head-coil. Rs-fMRI data were acquired for approximately 9 minutes using a T2*-weighted gradient-echo EPI sequence using the following parameters: TR/TE=2150/27ms; FOV=240x240x131mm, voxel size=3x3x3 mm; gap=0.3mm; flip angle=76.2°; dynamics=240. Rs-fMRI data were preprocessed using FSL with ICA-AROMA5 to detect and remove motion-related artifacts. Subsequently, dual-regression with variance normalization was applied using a predefined default-mode network mask6 with additional masks for white matter and CSF to regress out residual physiological noise components7. Non-parametric permutation testing (Randomise, 5000 permutations) was used to assess the association between SERT binding post-citalopram, blood plasma levels and DMN connectivity8. In addition, DMN connectivity differences between the three pre-treatment groups were assessed using a one-way analysis of variance (ANOVA), with follow-up post-hoc tests between the groups.Results
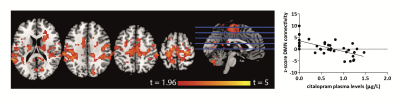
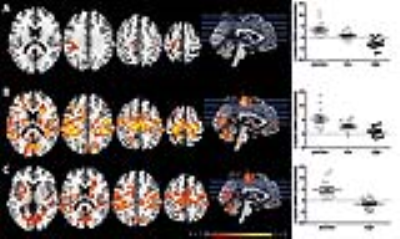
Higher SERT occupancy by the SSRI in the thalamus was significantly associated with decreased DMN connectivity with a number of cortical regions, including the anterior cingulate cortex (ACC), paracingulate gyrus, postcentral gyrus, superior parietal gyrus and temporal pole (Figure 1). Interestingly, we also observed that these brain regions show a negative correlation between blood-plasma levels and DMN connectivity (Figure 2). Mean DMN connectivity for each experimental group is shown in Figure 3. A one-way ANOVA showed a significant difference between the 3 groups (Figure 4A). Post-hoc tests showed a significant difference between the placebo and the high group (Figure 4C). However, the difference between the placebo and low group and placebo and high group failed to reach significance. Nevertheless, when analyzing the linear contrast of placebo > low > high we observe a pattern of differences in DMN connectivity (Figure 4B) comparable to the correlation with SPECT.Discussion and conclusions
Consistent with existing literature, we showed that citalopram decreased resting-state connectivity with the DMN. This is in line with previous studies that suggest that SSRIs normalize DMN patterns in depressed patients9. Additionally, we demonstrate that resting-state connectivity with the DMN is associated with thalamic SERT occupancy by citalopram, as well as with blood plasma citalopram levels. Thus, we here demonstrate that citalopram induces a dose-dependent reduction DMN connectivity. Interestingly, we observed that citalopram already affects DMN connectivity at a very low dose of 4 mg in which 40% of SERT is occupied. This suggests that DMN connectivity might be interesting biomarker, e.g. for treatment monitoring. Future studies in patients should confirm this, by studying the association between citalopram dose, DMN connectivity and treatment response.Acknowledgements
No acknowledgement found.References
1 Van der Ven, V., et al. (2013) Escitalopram Decreases Cross-Regional Functional Connectivity within the Default-Mode Network. PlosOne, 8(6):e68355
2 Meyer, J., et al. (2004) Serotonin Transporter Occupancy of Five Selective Serotonin Reuptake Inhibitors at Different Doses: An [11C]DASB Positron Emission Tomography Study. Am J Psychiatry, 161(5):826-835
3 Klein, N. et al. (2006) In vivo imaging of serotonin transporter occupancy by means of SPECT and [123I]ADAM in healthy subjects administered different doses of escitalopram or citalopram. Psychopharmacology (Berl) 188:263–72.
4 Booij, J., et al. (2007) Quantification of striatal dopamine transporters with 123I-FP-CIT SPECT is influenced by the selective serotonin reuptake inhibitor paroxetine: a double-blind, placebo-controlled, crossover study in healthy control subjects. J Nucl Med 48:359–366.
5 Pruim, R., et al. (2015) Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. NeuroImage, 112:278-287
6 Smith, S.M., et al. (2009) Correspondence of the brain's functional architecture during activation and rest. PNAS, 106(31):13040-13045
7 Beckmann C., et al. (2009) Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. NeuroImage, 47:S37-S39
8 Smith, S.M. & Nichols, T.E. (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1):83-98
9 van Wingen, G.A., et al. (2014) Short-term antidepressant administration reduces default mode and task-positive network connectivity in healthy individuals during rest. NeuroImage, 88:47-53
Figures