4653
Left and right temporal lobe epilepsies with mesial temporal sclerosis reveal distinct alterations in the intrinsic effective connectivity within the Papez circuit1Institute of Biomedical Engineering, National Taiwan University, Taipei, Taiwan, 2Institute of Medical Device and Imaging, College of Medicine, National Taiwan University, Taipei, Taiwan, 3Department of Neurology, National Taiwan University Hospital and College of Medicine, Taipei, Taiwan, 4Graduate Institute of Brain and Mind Sciences, College of Medicine, National Taiwan University, Taipei, Taiwan, 5Department of Medical Imaging, National Taiwan University Hospital and College of Medicine, Taipei, Taiwan, 6Molecular Imaging Center, National Taiwan University, Taipei, Taiwan
Synopsis
The present resting-state fMRI study performed structural equation modeling to evaluate intrinsic effective connectivity (iEC) within the Papez circuit in patients with unilateral temporal lobe epilepsy with mesial temporal sclerosis (TLE-MTS). Left TLE-MTS is characterized by decreased iEC on the left frontotemporal path, which might be associated with deficits in executive functions and working memory. Right TLE-MTS is characterized by decreased iEC on the paths in the right posterior limbic regions, which might be associated with deficits in autobiographical memory processing. Our findings might facilitate identifying potential epileptic network pathways and developing novel targeted therapies for unilateral TLE-MTS.
Introduction:
Previous structural1 and resting-state functional MRI2 (rsfMRI) studies on temporal lobe epilepsy with mesial temporal sclerosis (TLE-MTS) have demonstrated brain atrophy and abnormal functional connectivity in the Papez circuit. It is suspected that the Papez circuit might be a plausible network for seizure propagation. Furthermore, findings in neurophysiological3,4 studies imply that pathways within the Papez circuit could be characterized by directional connection conducting neural activity from one brain region to another, indicating intrinsically causal interactions between brain regions. Therefore, distinct alterations in the directional information flow within the Papez circuit in left and right TLE-MTS may highlight different pathophysiological changes and cognitive deficits in these two disease types. The present rsfMRI study performed structural equation modeling5 (SEM) to evaluate intrinsic effective connectivity (iEC) within the Papez circuit in patients with unilateral TLE-MTS. We hypothesized that compared with healthy controls, patients with unilateral TLE-MTS have altered iEC between brain areas of the Papez circuit, and left and right TLE-MTS show distinct patterns of altered iEC in the Papez circuit.Methods:
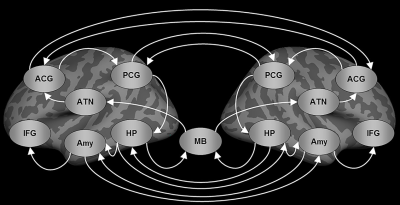
Subjects: Eighteen patients with left TLE-MTS (mean age=37.44±8.78 years), 18 patients with right TLE-MTS (mean age=39.22±9.45 years), and 37 healthy controls (mean age=37.86±8.89 years) were recruited in this study. MRI acquisition: All MRI data were acquired using a 3T MRI system (Tim Trio, Siemens) with a 32-channel phased-array head coil. T1W imaging was performed using a 3D MPRAGE sequence (TR/TE=2000/3 ms, flip angle=9°, FOV=256×192×208 mm, and matrix size=256×192×208. The 6-minute rsfMRI was performed using a gradient–echo EPI sequence (TR=2000 ms, TE=24 ms, flip angle=90°, FOV=256×256 mm, matrix size=64×64×34, slice thickness=3 mm). Data preprocessing: The rsfMRI data preprocessing was conducted using a MATLAB toolbox, namely DPARSF. The procedure included: slice timing correction, motion correction, registration of the T1W images to the rsfMRI data, brain segmentation of the T1W image, spatial normalization of the T1W image and rsfMRI data in the MNI space, spatial smoothing (6-mm FWHM Gaussian kernel), band-pass filtering (0.008–0.09 Hz), and nuisance regression. SEM analysis: 100 bootstrap iterative SEM calculations6 were performed to estimate the iEC on each of the 22 paths in the Papez circuit (figure 1). SEM started with selecting a set of ROIs and the directional path graph between the ROIs for constructing a model-implied data covariance matrix. The preprocessed rsfMRI BOLD signals extracted from the ROIs of the Papez circuit to construct an observed data covariance matrix for each participant. Observed data covariance matrices from a group of participants were averaged to produce a mean observed data covariance matrix. The maximal likelihood estimator was used to minimize the discrepancy between the model-implied data covariance matrix and the mean observed matrix to estimate path coefficients. The one-sample t test on 100 bootstrap iterative SEM calculations revealed that 19 of the 22 paths were significant in healthy controls, and these 19 paths were used to compare the differences in the iEC among the study groups. We performed the Kruskal–Wallis test to compare the path coefficients between healthy controls and patients with left and right TLE-MTS. The Mann–Whitney U test was performed as a post hoc test.Results:
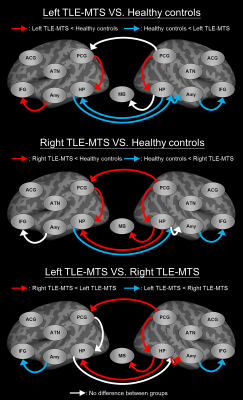
Compared with healthy controls, the two patient groups exhibited (figure 2): decreased iEC on the paths from the posterior cingulate gyrus (PCG) to the hippocampus (HP) in both hemispheres, increased iEC on the paths from the right amygdala (Amy) to the right inferior frontal gyrus (IFG), and increased iEC on the paths from the left HP to the right HP. Apart from the shared iEC alterations of the two patient groups, patients with left TLE-MTS showed distinctly decreased iEC on the paths from the left Amy to the left IFG, and increased iEC on the paths from the right HP to the right Amy and from the right HP to the left HP, and patients with right TLE-MTS showed distinctly decreased iEC on the two efferent paths of the right HP connecting the mammillary body and the left HP and one efferent path of the right PCG connecting the left PCG (figure 2).Discussion:
Left TLE-MTS is characterized by decreased iEC on the left frontotemporal path, which might be associated with deficits in executive functions and working memory7. Right TLE-MTS is characterized by decreased iEC on the paths in the right posterior limbic regions, which might be associated with deficits in autobiographical memory processing8.Conclusion:
Our findings might facilitate identifying potential epileptic network pathways and developing novel targeted therapies for left and right TLE-MTS.Acknowledgements
No acknowledgement found.References
1. Oikawa H, Sasaki M, Tamakawa Y, et al., The circuit of Papez in mesial temporal sclerosis: MRI. Neuroradiology. 2001 Mar;43(3):205-10.
2. Liu M, Concha L, Lebel C, et al., Mesial temporal sclerosis is linked with more widespread white matter changes in temporal lobe epilepsy. Neuroimage Clin. 2012 Oct 1;1(1):99-105.
3. Enatsu R, Gonzalez-Martinez J, Bulacio J, et al., Connections of the limbic network: a corticocortical evoked potentials study. Cortex. 2015 Jan;62:20-33.
4. MacLEAN PD, Psychosomatic disease and the visceral brain; recent developments bearing on the Papez theory of emotion. Psychosom Med. 1949 Nov-Dec;11(6):338-53.
5. Büchel C, Friston KJ, Modulation of connectivity in visual pathways by attention: cortical interations evaluated with structural equation modeling and fMRI. Cereb Cortex. 1997 Dec;7(8):768-78.
6. Lin FH, Agnew JA, Bellivwau JW, et al., Functional and effective connectivity of visuomotor control systems demonstrated using generalized partial least squares and structural equation modeling. Hum Brain Mapp. 2009 Jul;30(7):2232-51.
7. Giovagnoli AR, Relation of sorting impairement to hippocampal damage in temporal lobe epilepsy. Neuropsychologia. 2001;39(2):140-50.
8. Múnera CP, Lomlomdjian C, Gori B, et al., Episodic and semantic autobiographical memory in temporal lobe epilepsy. Epilepsy Res Treat. 2014;2014:157452.
Figures