4652
Optogenetically-initiated low frequency dorsal hippocampal activity enhances resting-state fMRI connectivity and visual memory retrieval performance1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China, 3Neurology and Neurological Sciences, Stanford University, Stanford, CA, United States, 4School of Biomedical Sciences, The University of Hong Kong, Hong Kong, China
Synopsis
Our recent study demonstrated that low frequency optogenetically-initiated hippocampal activities enhances brain-wide resting-state fMRI connectivity. However, the behavioral consequence of such connectivity enhancement remains unknown. Since hippocampus is known to play a prominent role in memory, we assessed the effects of such connectivity enhancement on short-term and long-term memory. Our experimental results demonstrated that, while low frequency dorsal hippocampus stimulation enhanced interhemispheric fMRI connectivity (in hippocampus, V1, A1 and S1), it also improved the long-term visual memory by enhancing memory retrieval (in contrast to memory encoding) performance.
Purpose
Functional connectivity measured by resting-state fMRI (rsfMRI) has been suggested to be an expression of brain-wide network behavior underlying cognitive functions1,2. Our recent study demonstrated that brain-wide functional connectivity was enhanced during and after low frequency optogenetic stimulation in dorsal dentate gyrus3 (dDG), a sub-region of the dorsal hippocampus (dHP). However, the behavioral consequence of such functional connectivity enhancement remains unknown. dHP plays a prominent role in memory4,5, especially memory encoding6, consolidation7 and retrieval6,8. However, the contribution of low frequency hippocampal-cortical activity initiated in dDG to memory functions is still unclear. In this study, we utilized low frequency optogenetic stimulation of dDG to enhance brain-wide functional connectivity. We then applied novel-object recognition (NOR) tests9,10 to assess the effects of low frequency dDG/dHP activities on short-term and long-term memory, as well as memory encoding and memory retrieval.Materials and Methods
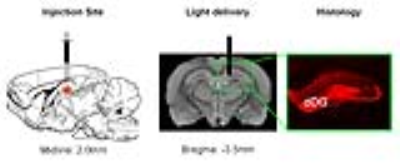
Animal preparation: AAV5-CaMKIIα::ChR2(H134R)-mCherry was injected to dDG of adult male SD rats (Fig. 1). After 6-weeks, an optical fiber was acutely or chronically implanted to deliver optogenetic stimulation for rsfMRI experiments or NOR tests, respectively. Note that NOR tests were applied 2-weeks after chronic fiber implantation. Also note that sham animals with fiber implantation but without ChR2 injection were used as controls for NOR.
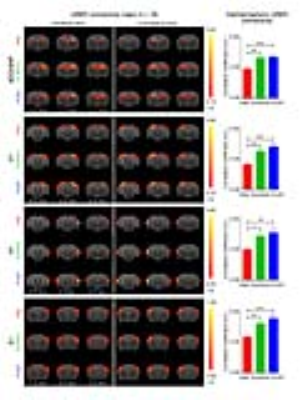
rsfMRI experiment and analysis: rsfMRI scans were performed before (PRE), during (DURING) and after (POST) optogenetic stimulation (n=18; Fig. 2). Note that DURING and POST were interleaved. Continuous 10-mins stimulation (473nm; 40mW/mm2; 1Hz; 10% duty cycle) was used to mimic low frequency hippocampal activities in dDG. rsfMRI data were acquired at 7T using GE-EPI (FOV=32×32mm2, matrix=64×64, α=50°, TE/TR=20/750ms). Standard preprocessing and seed-based analysis were applied to measure the bilateral functional connectivity in dorsal hippocampus (dHP), primary visual cortex (V1), primary auditory cortex (A1) and primary somatosensory cortex (S1).
Novel object recognition (NOR) behavioral tests and analysis: To assess low frequency dDG/dHP activities on general short-term and long-term memory, optogenetic animals (n=15) and sham controls (n=11) received low frequency (1 Hz) optogenetic stimulation for 10-mins prior to the start of acquisition, short-term memory and long-term memory phases (Paradigm-A; Fig. 3A). To further assess memory encoding and memory retrieval of long-term memory, optogenetic animals received stimulation prior to short-term memory phase (Paradigm-B, n=7; Fig. 3B) and long-term memory phase (Paradigm-C, n=9; Fig. 3C), respectively. In brief, animals were first habituated for 15-mins in the arena. The next day, animals were exposed to two identical objects for 3-mins during acquisition phase. 1-hr afterwards, animals were presented with the familiar object and a novel object for 3-mins to assess short-term memory. This assessment assumes animals would spend more time with the novel object if it could remember the familiar object. 24-hrs later, animals were again presented with one familiar and one novel object to assess long-term memory. Discrimination index11,12 (DI) was used to quantify the exploration preference for novel object. A positive DI indicates a preference for novel object.
Results and Discussion
Anatomical MRI scans and histology confirmed viral injection, fiber implantation, and ChR2-mCherry expression in dDG (Fig. 1). Interhemispheric functional connectivity of dHP, V1, A1 and S1 increased significantly DURING stimulation (Fig. 4; dHP: 37.3±7.4%, p < 0.01; V1: 55.6±11.5%, p < 0.01; A1: 44.2±6.9%, p < 0.05; S1: 43.3±9.0%, p < 0.01; one-way ANOVA followed by Bonferroni’s post-hoc test), and sustained POST stimulation (Fig. 4; dHP: 46.2±8.6%, p < 0.001; V1: 72.9±13.8%, p < 0.001; A1: 53.9±7.6%, p < 0.01; S1: 58.9±10.9%, p < 0.001; one-way ANOVA followed by Bonferroni’s post-hoc test). These results demonstrate that low frequency dDG stimulation enhanced interhemispheric functional connectivity in HP, V1, A1 and S1.
NOR results are shown in Fig. 5. Optogenetic animals under Paradigm-A and Paradigm-C spent significantly more time exploring the novel object (i.e., positive DI) during long-term memory phase, but not sham controls under Paradigm-A and optogenetic animals under Paradigm-B. During short-term memory phase, all groups displayed a preference for novel object (Fig. 5) as expected9,13. These results indicate that low frequency dDG stimulation improved long-term memory, in particular memory retrieval but not memory encoding.
In summary, low frequency dDG stimulation enhanced interhemispheric functional connectivity in HP, V1, A1 and S1, and such enhancement closely parallels the improved long-term memory, particularly the memory retrieval but not memory encoding. Our findings provide novel evidence on the “functions” of resting-state connectivity observed by rsfMRI. Furthermore, our approach here presents a new direction to modulate brain connectivity for potential behavioral improvements in normal and diseased brains.
Acknowledgements
This work was supported by the Hong Kong Research Grant Council (Grants C7048-16G and HKU17103015 to E.X.W.).References
- Deco, G., Jirsa, V. K. & McIntosh, A. R. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nature reviews. Neuroscience 12, 43-56, doi:10.1038/nrn2961 (2011).
- Greicius, M. Resting-state functional connectivity in neuropsychiatric disorders. Current opinion in neurology 21, 424-430, doi:10.1097/WCO.0b013e328306f2c5 (2008).
- Chan, R. W. et al. Low-frequency hippocampal-cortical activity drives brain-wide resting-state functional MRI connectivity. Proceedings of the National Academy of Sciences of the United States of America 114, E6972-E6981, doi:10.1073/pnas.1703309114 (2017).
- Scoville, W. B. & Milner, B. Loss of recent memory after bilateral hippocampal lesions. Journal of neurology, neurosurgery, and psychiatry 20, 11-21 (1957).
- Squire, L. R. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological review 99, 195-231 (1992).
- Zeineh, M. M., Engel, S. A., Thompson, P. M. & Bookheimer, S. Y. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science 299, 577-580, doi:10.1126/science.1077775 (2003).
- Ji, D. & Wilson, M. A. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nature neuroscience 10, 100-107, doi:10.1038/nn1825 (2007).
- Eldridge, L. L., Knowlton, B. J., Furmanski, C. S., Bookheimer, S. Y. & Engel, S. A. Remembering episodes: a selective role for the hippocampus during retrieval. Nature neuroscience 3, 1149-1152, doi:10.1038/80671 (2000).
- Liu, A., Jain, N., Vyas, A. & Lim, L. W. Ventromedial prefrontal cortex stimulation enhances memory and hippocampal neurogenesis in the middle-aged rats. eLife 4, doi:10.7554/eLife.04803 (2015).
- Leger, M. et al. Object recognition test in mice. Nature protocols 8, 2531-2537, doi:10.1038/nprot.2013.155 (2013).
- Lim, L. W. et al. Tetratricopeptide repeat domain 9A modulates anxiety-like behavior in female mice. Scientific reports 6, 37568, doi:10.1038/srep37568 (2016).
- Antunes, M. & Biala, G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cognitive processing 13, 93-110, doi:10.1007/s10339-011-0430-z (2012).
- Bruel-Jungerman, E., Laroche, S. & Rampon, C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. The European journal of neuroscience 21, 513-521, doi:10.1111/j.1460-9568.2005.03875.x (2005).
Figures