4651
Activating glucose transporter 2 positive neurons stimulates cerebral blood flow1École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2University of Geneva, Geneva, Switzerland, 3Center for Integrative Genomics, University of Lasuanne, Lasuanne, Switzerland
Synopsis
Glucose transporter 2 (Glut2)-positive cells are sparsely distributed in brain and play an important role in the stimulation of glucagon secretion in response to hypoglycemia. The effect on CBF of optogenetic activation of hypoglycemia responsive Glut2-positive neurons of the paraventricular thalamic area was measured in mice expressing channelrhodopsin2 under the control of the Glut2 promoter. Optogenetic activation of Glut2-positive neurons in the paraventricular thalamic nucleus induced a local CBF change similar in magnitude to the effect of hypoglycemia. Thus, our data indicate that brain Glut2-positive neurons are key regulators of hypoglycemia-induced activation of CBF.
Purpose
We aimed to demonstrate that activating specific Glut2-expressing neurons can evoke local CBF using the continuous arterial spin labeling (CASL) technique.Introduction
Glucose responsive neurons activated by hypoglycemia, referred to as glucose inhibited (GI) neurons, from the nucleus tractus solitarius (NTS) express the glucose transporter Glut2, provide GABAergic input to the dorsal motor nucleus of the vagus (DMNX) and are involved in stimulating vagal nerve activity and glucagon secretion linking hypoglycemia detection by the brain to the counterregulatory response (1). In addition, Glut2 neurons in the paraventricular thalamic nucleus (PVT) are glutamatergic and also activated by hypoglycemia. These PVT Glut2-postive neurons project excitatory inputs to the nucleus accumbens and when activated by hypoglycemia, Glut2 inactivation or optogenetics they increase motivated sucrose seeking behavior in mice (2).
Therefore, we hypothesis that simulating Glut2 neurons might evoke local cerebral blood flow (CBF). In this study, we used an optogenetic approach in combination with the continuous artery spin labeling (CASL) technique (3) to test the impact on regional blood flow of selectively activating Glut2-positive GI neurons in the PVT.
Methods
All experiments were carried out with the approval of the local Veterinary Office, and were conducted according to the federal and local ethical guidelines.
For the optogenetic study, we used mice expressing channelrhodopsin2 in Glut2-positive cells (Slc2a2-cre;Rosa26ChR2-YFP; mixed C57BL/6;SV129 background) (2).
Adult male mice (10-15 weeks) were used. Littermate animals were used as controls. One week before the experiment the mice were placed in a stereotaxic frame (David Kopf Instruments, U.S.A) under isoflurane anesthesia. A custom made optical cannula made of a ceramic ferrule (Precision Fiber Products) and an optical fiber (0.39 NA, 200 μm core diameter) was lowered in the PVT using the following stereotaxic coordinates: AP -0.4 / ML +0.8 / DV -3.4 mm with a 10° angle to avoid any damage to the superior sagittal sinus. The optical cannula was secured on top of the skull with tissue adhesive (VetBond; 3M) and dental cement (Paladur; Heraeus-Kulzer).
For MR experiments (9.4T), all mice were maintained within the targeted physiological range, i.e. 80-110 beats-per-minute and 36-37°C, by varying the percentage of isoflurane (i.e. 1-1.5%) and the temperature of circulating warm water, respectively. CBF was measured using CASL at 9.4T, as previously (3). In brief,16 pairs of 8-segmented semi-adiabatic SE-EPI images were acquired to map CBF (TE=42ms, FOV=23×15mm2, RO×PE=128×64, spectral width=200 kHz, 2.0 mm slice thickness). CBF maps, in units of mL/100g/min, were derived by pair-wise pixel-by-pixel calculation of label and control semi-adiabatic SE-EPI images as previously described (3).
The light stimulation protocol consisted of the delivery of 473 nm light pulses (10 ms light pulses at 20 Hz, 1 second on/1 second off, 15 mW) for 15 min during the acquisition of CBF measurements. CBF was monitored in anterior part of the PVT under basal and light-stimulated conditions. Basal CBF values were obtained by averaging CBF measurements before and after the optical stimulation.
Results and Discussion
To evaluate the role of specific Glut2-expressing
cells in the regulation of CBF, we
determined whether optogenetic activation of Glut2-neurons of the PVT could elicit a local increase in CBF. These
PVT Glut2 neurons have previously
been found to form a homogenous population of glucose-inhibited neurons that
control motivated sucrose seeking behavior (2). All mice were implanted with a fiber-optic cannula in the PVT. CASL
measurements were performed in the basal state and during optical stimulation. Light
stimulation of channelrhodopsin-expressing Glut2-neurons
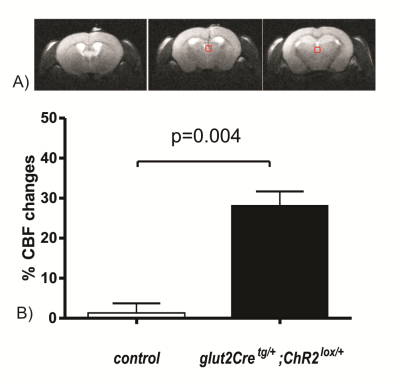
of the PVT increased local CBF in the PVT by 28±4% (Figure 1); no such increase
was observed in control mice not expressing channelrhodopsin (1±3%, Figure 1).
In this study, we used mice expressing channelrhodopsin2 in Glut2-expressing cells and we selectively activated those Glut2 neurons that are present in the PVT through stereotactic implantation of an optic fiber and light illumination of this region. We showed that this led to a robust increase in local CBF, similar in magnitude to that obtained following insulin-induced hypoglycemia. These PVT Glut2 neurons have previously been described to control motivated feeding behavior through their projection to the nucleus accumbens (2). Given the very similar CBF increases in PVT upon mild hypoglycemia and feeding behavior with genetic variant in Glut2 were observed in human, a dual function for these neurons in feeding behavior and CBF regulation would be compatible with the observation that they display very rich local arborization in addition to the high density projections to the nucleus accumbens.Thus, our data show that Glut2 expressing neurons are involved in control CBF.
Acknowledgements
This work was supported by the Centre d'Imagerie BioMédicale of the University of Lausanne (UNIL), University of Geneva (UNIGE), Hôpitaux Universitaires de Genève (HUG), Centre Hospitalier Universtaire Vaudois (CHUV), and Ecole Polytechnique Fédérale de Lausanne (EPFL); and the Leenaards and Jeantet Foundations. The work in BT’s laboratory was supported by grants from the Swiss National Science Foundation (3100A0B-128657) and a European Research Council Advanced Grant (INSIGHT).References
1) Lamy, C.M., Sanno, H., Labouebe, G., Picard, A., Magnan, C., Chatton, J.Y., and Thorens, B. 2014. Hypoglycemia-Activated GLUT2 Neurons of the Nucleus Tractus Solitarius Stimulate Vagal Activity and Glucagon Secretion. Cell Metab 19:527-538.
2) Labouebe, G., Boutrel, B., Tarussio, D., and Thorens, B. 2016. Glucose-responsive neurons of the paraventricular thalamus control sucrose-seeking behavior. Nat Neurosci 19:999-1002
3) Lei, H., Pilloud, Y., Magill,
A.W., and Gruetter, R. 2011. Continuous arterial spin labeling of mouse
cerebral blood flow using an actively-detuned two-coil system at 9.4T. Conf Proc IEEE Eng Med Biol Soc
2011:6993-6996.
Figures