4641
Brain states govern resting state functional connectivity dynamics1Institute of Microscopic Anatomy and Neurobiology, Mainz University, Mainz, Germany
Synopsis
A key aspect governing spatio-temporal activity patterns in fMRI is the brain state, particularly in animals being imaged mostly during sedation or anesthesia. Two main brain states have been recently identified in rodents[1], a persistent state similar to awake conditions, and a slow wave state characterized by spontaneous slow oscillation-associated slow wave activity. We analyzed the brain functional connectivity using spontaneous BOLD recordings in rats during these different states. We found that both states lead to differential functional connectivity patterns that can be clearly dissociated. These results are crucial for interpreting rodent studies in the framework of translational resting state research.
Introduction
Resting state fMRI has become an important research tool. In humans, resting state studies are performed in awake individuals. In contrast, in animals most of these studies are carried out under anesthesia or sedation, which can dramatically modify global cortical connectivity [2]. Depending on the anesthetic regime, two states can be characterized: a persistent state similar to awake conditions and a slow wave state characterized by spontaneous slow oscillation-associated slow wave activity, naturally occurring in slow-wave sleep. Recently, we have shown that slow wave state is reflected by characteristic whole-brain BOLD activity patterns that are not present in persistent state[1]. However, the underlying functional connectivity signatures in these two states remained unexplored. Here, by comparing resting state BOLD functional connectivity under persistent and slow wave states, we demonstrate that these different brain states can be characterized by particular connectivity patterns.Methods
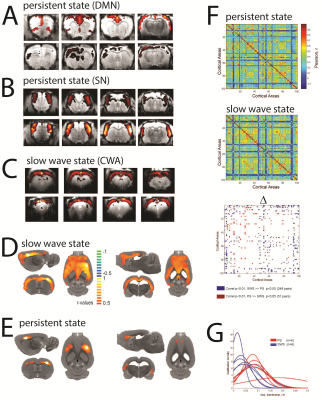
Functional MRI was performed using a 9.4 T Biospec scanner (Bruker) in 6 female Lewis rats that were anesthetized with isoflurane (1.2 %) to maintain slow wave state, or sedated by medetomidine to maintain persistent state (bolus injection of 0.04 mg/kg and constant perfusion of 0.08 mg/kg/hr). Each experiment consisted of 30 minutes of resting state recordings, flanked by phases of stimulation (each 30 minutes), subdivided into 15 minutes of visual stimulation, followed by 15 minutes of forepaw stimulation, to assure that the animal is the corresponding brain state, based on the pattern of stimulus-evoked response: localized in the respective cortical area signified persistent state, cortex-wide activation signified slow wave state. Anatomical images and T2*-weighted images were acquired. Functional data was preprocessed using Brain Voyager 20.6 (Brain Innovations). ICA was performed with SPM8. Connectivity analysis was performed with an in-house customized matlab (the mathworks) script using as template the Valdés-Hernández MRI template for rats[3].Results
Resting state ICA showed clearly distinct network activation patterns in persistent state compared with slow wave state. In the absence of sensory stimuli, during persistent state canonical default mode network activation can be observed (Figure 1A). Furthermore, also components involving somatosensory networks activations appear (Figure 1B). In contrast, under slow wave state, the main component observed corresponded to a cortex wide activation that most likely reflects slow wave activity(Figure 1C), as previously demonstrated[1]. In line with these findings, ROI based connectivity showed in slow wave state spatially more extended correlations with the rest of the cortex than in persistent state, both using somatosensory cortex (Figure 1D) and hippocampus (Figure 1E)as ROIs. To check if this reflects a general connectivity property of both states we used the Valdés-Hernández MRI template for rats[3] to assess the general functional connectivity of 100 cortical regions. This revealed a larger number of significant correlations between cortical regions in the slow wave (246 pairs) than in the persistent state (51 pairs). Furthermore, when analyzing the peak of the low frequency fluctuations in both states, the distribution of the cortical ROIs showed a significantly lower frequency for the slow wave compared with the persistent state (Figure 1F).Discussion
Functional connectivity analyses reveals characteristic differences in the cortical activity patterns in persistent and slow wave states. ICA shows a compartmentalized networks activity in persistent state but a cortex wide main component in slow wave state, suggesting a differential cortical connectivity. Along the same lines, when ROIs are used as seed, the correlation with the rest of the cortex under persistent state is quite restricted to particular networks, whereas in the slow wave state this correlation extends to almost the entire cortex. These results suggest a strong cortex-wide functional coupling of brain activity in slow wave state. Finally, in slow wave state low amplitude fluctuations of BOLD signal are significantly lower in frequency, suggesting a putative mechanism by which the cortex engages into a more synchronized activity and less compartmentalized networks than under persistent state.Conclusion
Our results demonstrate that persistent and slow wave state exhibit drastically different cortical connectivity patterns. In slow wave state network compartmentalization decreases considerably and BOLD signal frequency decreases. These results strongly suggest that the impact of brain states should be taken into account upon interpreting anesthesia based rodent studies in the framework of translational resting state studies. “Resting state” in the human setting, conducted in the resting but awake subject, corresponds to “persistent state” in terms of network activity patters. Consequently, we need to move towards brain-state-informed approaches in both animal and human fMRI to be able to compare spontaneous activity patterns across species, maximizing translational potential of resting state fMRI.
Acknowledgements
No acknowledgement found.References
1. Schwalm, M., et al., Cortex-wide BOLD fMRI activity reflects locally-recorded slow oscillation-associated calcium waves. Elife, Sep 15;6.
2.Liu, X., et al., The change of functional connectivity specificity in rats under various anesthesia levels and its neural origin. Brain Topogr, 2013. 26(3): p. 363-77.
3. Valdes-Hernandez, P.A., et al., An in vivo MRI Template Set for Morphometry, Tissue Segmentation, and fMRI Localization in Rats. Front Neuroinform, 2011. 5: p. 26.
Figures