4638
Optimization of motor fMRI activation in the setting of brain tumors via temporal resampling applied to the canonical HRFShruti Agarwal1, Jun Hua2,3, Haris I. Sair1, Sachin K. Gujar1, Scott Faro1, Hanzhang Lu2,3, and Jay J. Pillai1,4
1Division of Neuroradiology, Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Division of MR Research, Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3F. M. Kirby Research Center For Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 4Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
BOLD fMRI, which is an indirect measure of neuronal activity, involves several seconds offset in both initiation and cessation of the microvascular response with respect to actual timing of neural activity. In this study, we propose resampling of the canonical hemodynamic response function (HRF) to account for subject-wise temporal variability in BOLD responses in task paradigms. We demonstrate that temporal resampling of the canonical HRF may allow recapturing of lost signals in motor task activation maps (task fMRI). Further, it can also mitigate the effects of neurovascular uncoupling (NVU) in the sensorimotor network in patients with perirolandic gliomas.
Purpose
Blood Oxygen Level Dependent functional magnetic resonance imaging (BOLD fMRI) is an indirect measure of neuronal activity. BOLD fMRI detects alterations in deoxyhemoglobin concentration related to the hemodynamic response to neuronal activity. There is several seconds delay in the microvascular response with respect to the onset of neural activity. Furthermore, the microvascular response extends beyond the presentation of the task stimulus due to the phenomenon called mental chronometry.1,2 In the setting of neurovascular uncoupling (NVU), there is disruption of neuronal activity and adjacent microvascular responses in the vicinity of focal brain lesions, 3 which may further exacerbate this temporal offset and thus result in spuriously decreased ipsilesional BOLD activation in the absence of any attributable neurological deficit. In this study, we propose a method for temporal resampling of the canonical HRF may allow recapture of lost signals in motor task activation maps (fMRI) by accounting for subject-wise temporal variability of the microvascular response. Furthermore, it may also help to mitigate the effects of NVU in the sensorimotor network in patients with perirolandic tumors.Methods
Twelve de novo brain tumor patients who underwent clinical fMRI exams including task fMRI on a 3T MRI system were included in this IRB-approved study. Each patient displayed decreased/absent fMRI activation in the primary ipsilesional sensorimotor cortex in the absence of corresponding motor deficit or suboptimal task performance, consistent with NVU. 4 Imaging was performed on a 3.0 T Siemens Trio MRI with a 12-channel head matrix coil using a 3D T1 MPRAGE sequence for structural imaging and multiple 2D GE-EPI T2* weighted BOLD sequences for functional MR imaging (TR 2000ms/TE 30 ms/ 64x64x33 matrix). Task fMRI paradigms include a vertical tongue movement (TM) task and a bilateral simultaneous sequential finger tapping (FingM) task (each 3 minutes duration with alternating 30 second blocks of movement and rest, beginning with rest). SPM12 was used for preprocessing of fMRI data (slice timing correction, realignment, normalization to MNI space at 2mm voxel resolution, and spatially smoothing using a 6 mm FWHM Gaussian kernel). Z-score maps for the motor tasks were obtained from the general linear model (GLM) analysis using standard SPM canonical HRF (reflecting motor activation vs. rest). Pre- and post- central gyri were automatically parcellated using an Automated Anatomical Labeling (AAL) template5,6 for each patient. CL (contralesional) and IL (ipsilesional) ROIs circumscribing the combination of pre- and post- central gyri (CG) were obtained for each slice. The average time course (TC) of voxels in CL ROI in fMRI activation maps was obtained (see plot in Fig 1). In the proposed modified temporal resampling approach, we considered the first inflection point (P) from the resting baseline (see plot in Fig 1) as the beginning of the resting/control block. Hence, the modified duration of control block is (T-Δt) alternating with (T+Δt) seconds of task block repeated for 3 cycles. The fMRI response thus determined correlates well with task stimulus presentation time. Motor activation maps (both pre-correction and post-correction) were further analyzed using Amplitude Measured as a Percentage of Local Excitation (AMPLE) thresholding of 60% (i.e., only voxels with Z scores above 60% of a local cluster Z score maximum were considered “active”)7.Results
Motor activation maps for two patients obtained pre-correction (i.e. before temporal resampling), post-correction (i.e. after temporal resampling) and their difference map are depicted in Figures 1 & 2. Among the 12 cases, the algorithm suggested time resampling for 9 cases (i.e. 75% cases have improved activation followed by the temporal resampling correction).The number of voxels in the contralesional (CL) and ipsilesional (IL) ROI for each subject pre- and post- correction was obtained (see Table 1) and a group analysis was performed which reveals significantly increased number of voxels displaying contralesional motor cortical activation post-correction compared to pre- correction (p=0.032 using paired t-test). Group analysis also revealed that there is a trend level significant increase (p=0.059) in the number of activated voxels in post-corrected ipsilesional perirolandic ROIs in these patients with motor cortical NVU.Discussion
In this preliminary study we demonstrate the feasibility of optimization of primary motor cortical activation in the setting of brain tumors through use of a correction algorithm based on novel temporal resampling of the canonical HRF.Conclusion
In patients with brain tumors, our novel correction algorithm based on temporal resampling applied to a canonical HRF demonstrates enhanced clinically relevant sensorimotor activation in bilateral cerebral hemispheres.Acknowledgements
This work is partially supported by NIH grant R42 CA173976-02 (NCI).References
- Posner MI. Chronometric Explorations of Mind. London: Oxford Univ. Press; 1978.
- Georgopoulos AP & Pellizzer G. Neuropsychologia. 1995;33:1531–1547.
- Attwell D, et al. Nature 2010;468:232-243
- Zacà D, et al. J Magn Reson Imaging 2014;40(2):383-90
- Tzourio-Mazoyer N et al. Hum Brain Mapp 2002;17:143–55
- Smith SM. Hum Brain Mapp 2002;17:143–55
- Voyvodic et al.,JMRI 2009; 29(4):751-9.
Figures
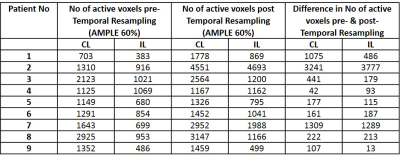
Table
1:
This table lists number of
suprathreshold (active) voxels in the primary sensorimotor cortex within the
ipsilesional (IL) and contralesional (CL) regions of interest (ROI) for each
patient. The second and third columns describe the number of active voxels
prior to application of the temporal resampling correction (for CL and IL,
respectively), while the fourth and fifth columns list the number of active
voxels following correction. The last two columns list the number of voxels in
the difference maps (post-correction minus pre-correction) for CL and IL ROIs.
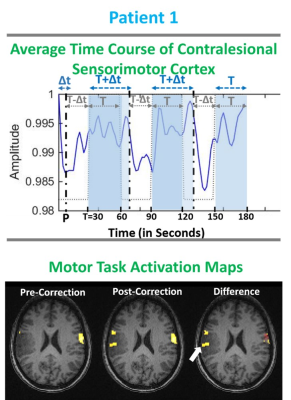
Figure 1: Patient 1 with grade II oligodendroglioma.
Top row shows average time course of primary sensorimotor cortex during the TM
task. Bottom row displays the TM fMRI activation map overlaid on T1 3D MPRAGE
structural images. Pre-corrected (i.e. prior to temporal resampling of
canonical HRF) motor activation map (Z-score>3.0) displays abnormally
decreased activation compared to the contralesional sensorimotor cortex.
Post-corrected motor activation map shows newly detected activation in the
right primary motor cortex. The difference map between the post- and pre-correction
maps displays the newly detected activation with arrow pointing to the enhanced
detection of voxels in tumor affected regions.
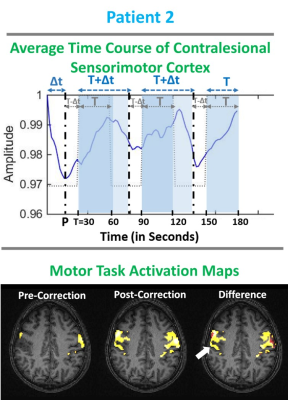
Figure 2: Patient with grade II oligoastrocytoma.
Top row shows average time course of primary sensorimotor cortex during the TM
task. Bottom row displays the TM fMRI activation map overlaid on T1 weighted 3D
MPRAGE structural images. Pre-corrected (i.e. prior to temporal resampling of
canonical HRF) motor activation map (Z-score>3.0) displays abnormally
decreased ipsilesional activation compared to contralesional sensorimotor
cortex. Post-corrected motor activation map displays newly detected activation
in the right primary motor cortex. The difference map between the post- and
pre-correction maps displays the newly detected activation with arrow pointing
to the enhanced detection of voxels in tumor affected regions.