4637
Optimization of breath hold cerebrovascular reactivity mapping (BH CVR) at ultra-high field through temporal correction of the hemodynamic response functionShruti Agarwal1, Jun Hua2,3, Haris I. Sair1, Sachin K. Gujar1, Hanzhang Lu2,3, and Jay J. Pillai1,4
1Division of Neuroradiology, Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Division of MR Research, Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3F. M. Kirby Research Center For Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 4Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Cerebrovascular reactivity (CVR) provides clinical insight into vascular health and is useful for identifying cortical regions affected by neurovascular uncoupling (NVU). BOLD imaging using breath-hold (BH) tasks can be effectively used for CVR mapping. At 7T, spatial specificity can be improved relative to standard 3T imaging, but optimization of CVR maps is often problematic. We propose temporal correction of the theoretical hemodynamic response function (HRF) to account for subject-wise temporal adjustment of the average respiration-induced hemodynamic response to a BH task to optimize 7T BH CVR maps in cases of poor patient task compliance.
Purpose
Cerebrovascular reactivity (CVR) can be useful for identification of cortical regions affected by neurovascular uncoupling (NVU).1 CVR is defined as the change in cerebral blood flow (CBF) per unit change in a vasoactive stimulus. CVR mapping can be accomplished using BOLD imaging with a breath hold (BH) task. BOLD image contrast mechanisms differ at conventional (≤ 3T) and ultrahigh (≥ 7T) fields. At 7T, BOLD signal more closely reflects the concentration of deoxyhemoglobin in capillaries rather than in larger veins (unlike at 3T), and therefore, spatial specificity is improved since cortical neuronal activity is more closely linked to blood flow changes in the microvasculature than in larger vessels.2,3 In this study, we propose a temporal correction of the theoretical hemodynamic response function (HRF) for optimization of 7T BH CVR maps in light of subject-wise temporal variability of the average respiration-induced hemodynamic response to a BH task.Methods
Fourteen patients with de novo primary brain tumor referred for routine 3T clinical presurgical fMRI mapping at our institution also underwent a ultra-high field (7T) fMRI study on the same day or at most within three weeks of the clinical fMRI scan and prior to surgical resection or initiation of chemoradiation therapy. This study was approved by our Institutional Review Board. Imaging was performed on a 3.0 T Siemens Trio MRI with a 12-channel head matrix coil using a 3D T1 MPRAGE for structural imaging and multiple 2D GE-EPI T2* weighted BOLD sequences for task functional imaging. 7T scanning was performed using research sequences on a 7.0T Philips MRI system with a 32-channel head matrix coil using a 3D T1 3D MPRAGE imaging sequence for structural imaging and multiple 2D fast echo planar imaging T2*-weighted BOLD sequences for functional imaging. The breath hold (BH) task includes normal breathing period of 40 seconds followed by a 4 second block of slow controlled inspiration that immediately preceded each 16-second BH period.4 This cycle was repeated four times and at the end of the last BH period an additional normal breathing period of 20 seconds was added. SPM12 software was used for preprocessing of BH data (slice timing correction, realignment, normalization to MNI space at 2mm voxel resolution, and spatially smoothing using a 6 mm FWHM Gaussian kernel). Z-score maps for BH tasks were obtained using general linear model (GLM) analysis using a theoretical HRF5 reflecting hypercapnia vs. baseline. Cortical regions in the contralesional hemisphere were automatically parcellated using an Automated Anatomical Labeling (AAL) template6 for each patient. The average time course (TC) of contralesional voxels in the 7T BH CVR map was obtained (see plot in Fig 1). Goodness of fit between peaks in the TC plot and those in the theoretical HRF (see black dotted curve in the plot) using root mean square error (RMSE) was calculated for various time shifts of the HRF to obtain the optimal time shift where the best fit occurs according to least RMSE. This time shift was considered as the optimal temporal correction for the HRF. GLM analysis was then performed using the temporally corrected HRF. The obtained corrected 7T BH CVR maps were then compared with 3T BH CVR maps. Four 3T cases and one 7T case were discarded due to motion degraded BH data. Of the remaining nine cases, six cases (67%) demonstrated substantial improvement in the BH CVR map quality following application of this HRF temporal correction method.Results
7T BH CVR maps for a patient obtained pre-correction (i.e. before application of the temporal HRF correction algorithm) and post-correction (i.e. after temporal HRF correction) are depicted along with the corresponding 3T BH CVR map in Figure 1. Table 1 depicts the RMSEs between the time courses and the theoretical HRF which was used for GLM analysis of 3T BH, uncorrected 7T BH and temporally corrected 7T BH data. The RMSE reveals the superior goodness of fit following application of temporal correction of the theoretical HRF.Discussion
In this preliminary study we have demonstrated the feasibility of optimization of 7T BH CVR maps in the setting of brain tumors through use of a correction algorithm based on novel temporal correction of the standard theoretical HRF described by Birn and colleagues.Conclusion
In patients with brain tumors, application of our novel HRF optimization method based on temporal correction of the HRF provides superior quality BH CVR maps at ultra-high field.Acknowledgements
This work was supported by a Johns Hopkins Univ. Brain Science Institute grant and partially by NIH grant R42 CA173976-02 (NCI).References
- Pillai JJ, et al. AJNR Am J Neuroradiol 2015;36(1):7-13.
- Thulborn KR, et al. Biochem Biophys Acta 1982;714:265–270.
- Siero JCW, et al. PLOS One 2013;8(1) e354560:1-8.
- Zacà D, et al. J Magn Reson Imaging 2014;40(2):383-90.
- Birn RM, et al. Neuroimage 2008;40:644-654.
- Tzourio-Mazoyer N, et al. Hum Brain Mapp 2002;17:143–55
Figures
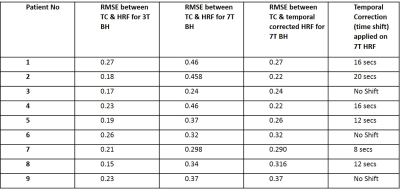
Table 1: Root mean square errors (RMSEs) between the
theoretical HRF used to model average respiration-induced hemodynamic response
to a BH task and the average time course (TC) of the voxels in contralesional holohemispheric
cortex during performance of the BH task at both 3T and 7T are tabulated
(columns 2 & 3). RMSE between temporally corrected HRF and TC at 7T and the
time shifts applied to obtain the temporally corrected HRF for each of the 9
cases are also tabulated (columns 4 & 5). This reveals the goodness of fit among
all three HRFs used to obtain BH CVR maps.
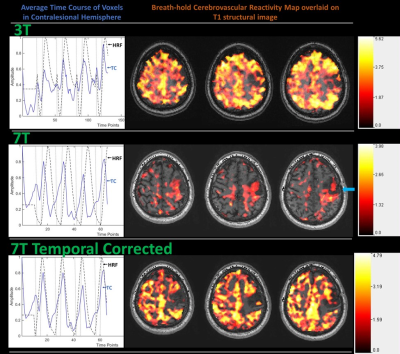
Figure 1: Patient with grade II oligodendroglioma.
Top row displays average time course (TC) of contralesional hemisphere during
the breath hold (BH) task performed at 3T along with theoretical hemodynamic
response function (HRF) (dashed black line in the plot) and BH CVR maps
obtained using this HRF overlaid on T1 weighted 3D MPRAGE structural images. The
middle row displays the same for 7T BH data. The arrow points to the poor fit
of the theoretical HRF. The last row displays the temporally corrected HRF and
7T BH CVR maps obtained using this temporally corrected HRF.