4635
The interaction between neural and autonomic correlates of stress: a pilot study measuring simultaneous heart rate variability and BOLD fMRI1Translational Research Center, University Hospital of Psychiatry and Psychotherapy, Bern, Switzerland
Synopsis
Stress has been associated with autonomic arousal as well as functional alterations in frontal and limbic regions which are important in the regulation of autonomic activity. Latter can be indexed by heart rate variability (HRV). In the present pilot study simultaneous BOLD fMRI and HRV were measured during the performance of a stress test. In three healthy subjects stress, relaxation and paced breathing were associated with frontal and limbic activation. More subjects need to be measured in order to strengthen the results and to integrate them as neural markers in an integrative and multilevel model of stress.
Introduction
The biological stress response has been associated with autonomic arousal as well as functional alterations in frontal and limbic brain regions 1. A convenient measure of autonomic regulation is heart rate variability (HRV), which is also supposed to mirror the strength of the interaction between the prefrontal cortex (PFC) and the limbic system 2. The PFC may regulate autonomic arousal by inhibitory control. Stress is thought to cause a disinhibition of this control, which in turn leads to autonomic arousal, i.e. sympathetic dominance and parasympathetic withdrawal 3. Studies measuring fMRI and autonomic activity indexed by HRV in healthy individuals are scarce 4. In the present pilot study simultaneous BOLD fMRI and HRV were measured during the performance of a stress test in order to investigate the interaction between neural and autonomic activity during stress.Methods
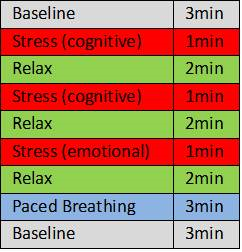
Three healthy individuals performed a block design stress test involving cognitive and emotional tasks, relaxation periods and paced breathing at the rate of 6 breaths/ min, which is known to increase parasympathetic activity (Fig.1). For HRV, an electrocardiogram was measured with a MR-compatible monitoring system (Philips Expression IP5). HRV data were analyzed using the Kubios HRV Premium software version 3.0.2. The ECG signal was divided in 1-minute-samples and for each the parasympathetic measure root mean square of successive differences (RMSSD) was calculated. Imaging was performed in a 3T Siemens Magnetom Trio TIM system equipped with a 64-channel head coil. FMRI was performed with a BOLD T2*-weighted echo planar imaging (EPI) sequence (48 slices. TR/TE = 1000/30 ms, flip angle = 90°, slice thickness = 2.4 mm, inter slice gap thickness = 0 mm, matrix size = 94 × 94, field of view (FOV) = 230 mm × 230 mm, 1085 volumes in 18:10 min. In addition, structural images were collected using a three-dimension (3D) magnetization-prepared rapid gradient-echo (MP-RAGE) T1-weigthed sequence (repetition time (TR)=1950 ms, echo time (TE)=2.2 ms, 900 ms inversion time (Ti) and a flip angle of 9° (FA), field of view (FOV) 256x256 mm2, matrix dimension 256x256, resolution 1x1x1mm3. Images were realigned, coregistered to the T1 images, normalized into standard MNI space and smoothed with a 6mm FWHM Gaussian kernel. A GLM was calculated with the blocks of the stress test (convolved with boxcar HRF) and the HRV RMSSD values as predictors. We first performed a 1-level analysis building following contrasts: stress vs. relax, and baseline vs. paced breathing. Additionally, we performed a preliminary 2-level analysis with images of these contrasts by simple one-sample t-tests. All MRI data were analyzed using SPM12.Results
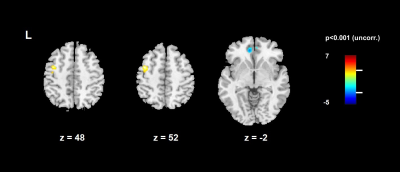
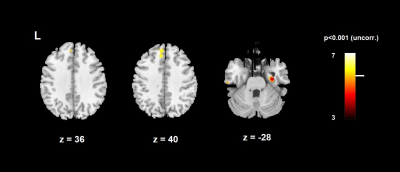
The 2-level analysis comparing stress and relax revealed a positive contrast - meaning that the condition stress minus relax is greater than 0 - in the left medial frontal cortex (BA6), i.e. the supplementary motor area (SMA), and a negative contrast in the left dorsal anterior cingulate cortex (dACC, BA32) (Fig. 2). Contrasting baseline and resonant breathing yielded positive effects in the superior frontal gyrus (dorsolateral PFC, BA9), and the right hippocampus (Fig. 3).Discussion
Integrating autonomic activity - indexed by HRV RMSSD - into the analysis of neural correlates of stress, resulted in the activation of frontal and limbic regions. The activation of the left SMA during the stress blocks may derive from speech production when communicating the results of the stress task. Thus, the activity of the SMA may refer to task performance rather than to autonomic arousal. The negative contrast in the dACC produced by the comparison of stress and relax blocks indicates a higher activation during relaxation. The dorsal ACC has been proposed to mediate interactions between cognitive processing and autonomic arousal5. Moreover, ACC is involved in anticipatory fear processing 6. As dACC was more activated during the relaxation periods, this result might indicate anticipatory arousal before the stress task. During baseline there was higher activation in both the BA9 and the right hippocampus compared the paced (resonant) breathing. The BA9 is associated with episodic memory retrieval. This function seems to be engaged during baseline, but not during paced breathing, as the breathing task requires attentional resources. Also the right hippocampus shows higher activation during baseline. The hippocampus is supposed to be part of the inhibitory areas, the activation of which is negatively correlated to HRV7. Thus, a reduced activity during the paced breathing task may mirror the parasympathetic activation induced by resonant breathing. Clearly, more subjects need to be measured in order to strengthen the results. Stress-related neural and autonomic markers as well as their interaction may be integrated into a multilevel model of stress.Acknowledgements
No acknowledgement found.References
1. Orosz A, Federspiel A, Haisch S, et al. A biological perspective on differences and similarities between burnout and depression. Neurosci Biobehav Rev. 2017 Feb;73:112-122.
2. Sakaki M, Yoo H, Nga L, et al. Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. Neuroimage. 2016 Oct 1;139:44-52.
3. Brosschot J, Van Dijk E, and Thayer J. Daily worry is related to low heart rate variability during waking and the subsequent nocturnal sleep period. Int J Psychophysiol. 2007 Jan;63(1):39-47. Epub 2006 Oct 3.
4. Lane R, McRae K, Reiman E, et al. Neural correlates of heart rate variability during emotion. Neuroimage. 2009 Jan 1;44(1):213-22.
5. Critchley H, Mathias C, Josephs O, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003 Oct;126(Pt 10):2139-52. Epub 2003 Jun 23.
6. Soravia L, Orosz A, Schwab S, et al. CBT reduces CBF: cognitive-behavioral therapy reduces cerebral blood flow in fear-relevant brain regions in spider phobia. Brain Behav. 2016 Jul 6;6(9):e00510. eCollection 2016 Sep.
7. Valenza G, Duggento A, Passamonti L, et al. Resting-state brain correlates of instantaneous autonomic outflow. Conf Proc IEEE Eng Med Biol Soc. 2017 Jul;2017:3325-3328.