4629
Investigation of the relationship between grey matter volume and cerebrovascular reactivity in aging1Physics, Concordia University, Montreal, QC, Canada, 2Université de Montréal, Montreal, QC, Canada, 3Concordia University, Montreal, QC, Canada, 4Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 5McGill University, Montreal, QC, Canada
Synopsis
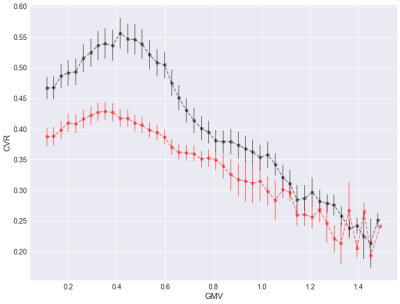
Cerebrovascular reactivity (CVR) is decreased in aging and disease, and is thought to reflect vascular health in the brain. Here, we explore the relationship between grey matter volume (GMV) and CVR in healthy younger and older adults. Results show that this relationship is more complex than previously thought, with a positive relationship between CVR and GMV when GMV is low, but a negative relationship at high GMVs both in younger and older adults. Future analyses will explore the role of cerebral blood flow in this relationship.
Introduction
Aging is known to be associated with grey matter (GM) atrophy [1], and these changes are thought to lead to functional deficits [2]. The vasculature throughout the body is also affected by the aging process and it is thought that because it is a high flow organ, the brain’s vasculature may be particularly sensitive to damage even in healthy aging [3, 4]. While it has been proposed that vascular damage may precede and in some cases cause GM atrophy, the relationship between GM atrophy and vascular health is still unclear [3]. Vascular health can be measured using several MRI techniques, and recent studies have proposed that cerebrovascular reactivity (CVR) measured using the blood oxygen level dependent (BOLD) signal response to a hypercapnia challenge may be a sensitive marker of poor cerebrovascular health and vessel elasticity in the brain [4]. Previous studies have shown CVR to be decreased in aging [5, 6], and in several diseases [7, 8]. Here, we explore the relationship between GM atrophy and CVR in older adults to investigate the use of CVR as a cerebrovascular health marker.Methods
Acquisitions were conducted in 31 young (10 female, 24 ± 3 years) and 55 older (37 female, 64 ± 5 years) healthy participants on a 3T MRI system. Sessions included an anatomical, 1mm3 MPRAGE acquisition (TR/TE/flip angle = 2300ms/3ms/90°) and a pseudo-continuous arterial spin labeling (pCASL) run, providing simultaneous BOLD contrast using dual-echo readouts (TR/TE1/TE2/flip angle = 2000ms/10ms/30ms/90° with 4x4x7mm voxels, and 11 slices, post-label delay=900ms, tag duration=1.5s, with a 100mm gap) during a hypercapnia challenge (5mmHg end-tidal CO2 change target, iso-oxic during two 2min blocks). BOLD percent effects were determined in each voxel and divided by the change in end-tidal CO2 concentration obtained in each participant during the hypercapnia manipulation to calculate CVR.T1-weighted anatomical images were segmented with the CAT12 toolbox (http://www.neuro.uni-jena.de/cat/) of SPM12 to produced modulated and smoothed (4mm) maps of grey matter volume (GMV). CVR slabs for each individual were registered to the corresponding T1w images (rigid) and transformed to the group GMV space (non-linear, unmodulated) for comparison. The intersection of mean GMV and CVR (threshold >= 0.10) was used to identify overlapping voxels for analysis. We investigated the relationship between CVR and GMV by binning and then averaging voxel-wise GMV into 50 bins and extracting the corresponding bin mean CVR for each participant. Group averages and standard errors were plotted to identify the relationship. To further determine the spatial pattern of the relationship, we also performed voxel-wise correlations between CVR and GMV across all participants.Results
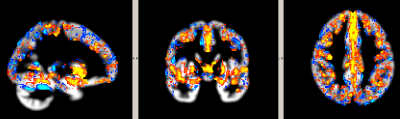
The relationship between CVR and GMV was found to be positive in voxels with low GMV, and negative in voxels with larger GMV (Fig. 1). Regions with positive and negative relationships between CVR and GMV are shown in Fig. 2.Discussion
A simple interpretation of CVR as a cerebrovascular health biomarker would lead to the expectation that CVR would have a linear and positive relationship with GMV. Here, we show that this relationship is more complex than previously thought. This finding is, however, compatible with studies showing both positive and negative relationships between fitness and CVR [4]. The complexity in this relationship may make interpretations of CVR as a cerebrovascular health biomarker more complicated in healthy populations such as the one used in this study.Future work will include examining how the role of cerebral blood flow (CBF) contributes to this relationship by including an analysis of the ASL data included in this study, and examining the relationship with cortical thickness.Conclusion
While CVR has been shown to be decreased in aging and disease, the relationship between CVR and GMV shows that CVR may not be a simple biomarker of cerebrovascular health in healthy populations. The complex relationship between GMV, CVR and other indicators of vascular health, such as fitness, means that future investigation are needed to understand the meaning of CVR as a biomarker of vascular health.Acknowledgements
The authors thank Carollyn Hurst and André Cyr for their help with data acquisition, Mélanie Renaud, Cécile Madjar and Élodie Boudes for their help with logistics. They thank Jiongjiong Wang of the Department of Neurology at UCLA who provided the dual-echo pseudo-continuous arterial spin labeling sequence. This work was supported by the Canadian Institutes of Health Research (MOP 84378), the Canada Foundation for Innovation (Leaders Opportunity Fund 17380), the Ministère du développement économique, de l’innovation et de l’exportation (PSR-SIIRI-239), the Canadian National Sciences and Engineering Research Council (R0018142, RGPIN-2015-04665) and the Heart and Stroke Foundation of Canada (N.I.A. C.J.G.).References
1. Salat, D.H., et al., Thinning of the cerebral cortex in aging. Cereb Cortex, 2004. 14(7): p. 721-30.2.
2. Gorbach, T., et al., Longitudinal association between hippocampus atrophy and episodic-memory decline. Neurobiol Aging, 2017. 51: p. 167-176.3.
3. Chen, J.J., H.D. Rosas, and D.H. Salat, Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage, 2011. 55(2): p. 468-78.4.
4. Gauthier, C.J., et al., Hearts and minds: linking vascular rigidity and aerobic fitness with cognitive aging. Neurobiol Aging, 2015. 36(1): p. 304-314.5.
5. Gauthier, C.J., et al., Age dependence of hemodynamic response characteristics in human functional magnetic resonance imaging. Neurobiol Aging, 2013. 34(5): p. 1469-85.6.
6. De Vis, J.B., et al., Age-related changes in brain hemodynamics; A calibrated MRI study. Hum Brain Mapp, 2015. 36(10): p. 3973-87.7.
7. Anazodo, U.C., et al., Impaired Cerebrovascular Function in Coronary Artery Disease Patients and Recovery Following Cardiac Rehabilitation. Front Aging Neurosci, 2015. 7: p. 224.8.
8. De Vis, J.B., et al., Calibrated MRI to evaluate cerebral hemodynamics in patients with an internal carotid artery occlusion. J Cereb Blood Flow Metab, 2015. 35(6): p. 1015-23.