4628
Age-modulated changes in cerebrovascular reactivity after a single bout of exercise in young adults1School of Physics and Astronomy, Cardiff University Brain Research Imaging Centre (CUBRIC), Cardiff University, Cardiff, United Kingdom, 2School of Psychology, Cardiff University Brain Research Imaging Centre (CUBRIC), Cardiff University, Cardiff, United Kingdom, 3Cardiff and Vale University Health Board, Cardiff, United Kingdom
Synopsis
Exercise is a potent trigger for both neurogenesis and vascular plasticity yet the underlying temporal dynamics are not known. We aimed to test whether a single 20-minute bout of aerobic exercise was sufficient to induce changes in cerebrovascular reactivity (CVR) to carbon dioxide in young healthy adults using a dual-echo pulsed ASL sequence with a hypercapnia challenge. We show that age modulates the change in CVR following exercise, and strongly predicts baseline CVR, independent of resting physiology and fitness. Our findings motivate the further exploration of the role of age in exercise-induced vascular plasticity.
Introduction
With exercise a potent trigger for both neurogenesis and vascular plasticity, identifying the underlying temporal dynamics is vital in order to exploit the vast therapeutic potential. There is evidence that during, and immediately after, exercise, cerebral autoregulation in large vessels is temporarily disturbed. Given that neurogenesis and angiogenesis are tightly coupled1, and structural remodelling of the limbic system has been demonstrated to occur rapidly after two hours of training2, here we investigated the timescale of vascular plasticity. Specifically, we aimed to test whether a single bout of aerobic exercise was sufficient to induce changes in cerebrovascular reactivity (CVR), a marker of vascular reserve.Methods
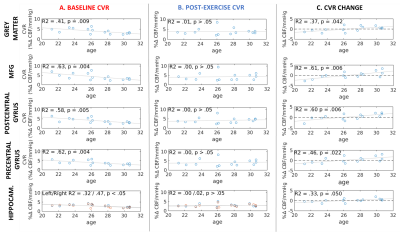
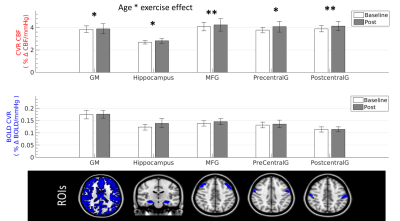
Baseline simultaneous BOLD and CBF data were acquired for 16 healthy normotensive participants (7 males, 26.7 ± 3.09 years old, range = 21-30 years) at 3T using a PICORE QUIPSS II3 dual-echo ASL sequence (14 slices, 64 spiral, TE1 = 2.7ms, TE2 = 29ms, TR = 2.4s, TI1 = 700ms, TI2 = 1.5s, FOV = 19.8cm, flip angle = 20°, slice thickness/gap 7/1.5mm) . Hypercapnia (+7-8 mmHg PETCO2 above resting levels) was induced in a block-design fashion with alternating 120-s blocks of normocapnia and hypercapnia. Participants then completed 20-minutes of aerobic exercise on a cycle ergometer outside of the scanner, before being immediately re-scanned, with the dual-echo sequence acquired at approximately 25-minutes post-exercise (10-min acquisition time). Image time-series were motion corrected and brain masked4 and CBF time-series were calculated from the first echo by subtracting tag and control pairs following interpolation to the TR. A BOLD weighted time-series was calculated from the second echo and tag and control pairs were averaged. CBF was quantified using a single compartment model3. The PETCO2 trace was convolved with a single haemodynamic response function and fitted to the CBF and BOLD image time-series in each subject to obtain CVR, measured as %CBF or %BOLD signal change per mmHg PETCO2 change. CVR was assessed globally in the grey matter (GM) and in 4 ROIs: the hippocampus, middle frontal gyrus (MFG), precentral and postcentral gyrus (Fig.1). Maps were thresholded voxelwise based on the R2 of the fit. A repeated-measures ANCOVA, with age as a covariate, was used to assess the effect of exercise on CVR.Results
Age significantly predicted baseline CBF CVR in all ROIs, accounting for 32-63% of the variance in CBF CVR (Fig.1A). A significant interaction between age and CBF CVR was found in the grey matter and all of the ROIs (p < 0.05 FDR-adjusted, η2 > 0.25 indicating a large effect size, Fig.2). There were no main effects or interaction effects for BOLD CVR (all p > 0.05). Age did not predict CBF CVR post-exercise (Fig.1B), and the change in CBF CVR was significantly predicted by age in all regions (p < 0.05 FDR-adjusted). Despite the small age range in this young cohort, a negative change in CBF CVR was observed in younger participants, whilst a positive change was observed in older participants (Fig. 1C). The peripheral vascular response to hypercapnia was not modulated by age; the degree of hypercapnia induced at baseline and post-exercise did not differ (Baseline = +7.68 ± 0.27 mmHg; Post = +7.72 ± 0.48 mmHg; t12 = -0.076, p > 0.05) and was not correlated with age (r = -0.09 and -0.19 respectively, p > 0.05). Heart rate, respiration rate and mean arterial pressure did not change with hypercapnia at baseline or post-exercise (p> 0.05), and did not interact with age. Similarly, age did not correlate with baseline fitness (VO2 peak), body mass index, self-reported physical activity levels, resting heart rate, resting mean arterial pressure, resting PETCO2, or performance on the exercise intervention (heart rate reserve achieved, average lactate levels), all p > 0.05.Conclusion
Although CVR, a measure of vascular health, is known to be reduced during the normal ageing process5, the strong relationship with age is notable given the small age range in this young cohort; although a decreasing developmental CVR trajectory has been shown in a small sample of 15-30 year olds6. Age-related differences in resting peripheral physiology, or in the peripheral vascular response to C02, which may influence CVR, do not appear to explain the results. The differential effect of age on the CVR response to a single session of exercise in young healthy adults is novel and the role of age in exercise-induced vascular plasticity warrants further attention in a larger sample size and age range.Acknowledgements
We wish to acknowledge the Waterloo Foundation and Wellcome Trust [WT200804] for funding this work.References
1. Chopp, M., Gang Zhang, Z. & Jiang, Q. Neurogenesis, Angiogenesis, and MRI Indices of Functional Recovery From Stroke. Stroke. 2007;38:827-831
2. Sagi, Y. et al. Learning in the fast lane: new insights into neuroplasticity. Neuron 73, 1195–203 (2012).
3. Wong, E. C., Buxton, R. B. & Frank, L. R. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). Magn. Reson. Med. 39, 702–708 (1998).
4. Cox, R. W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–73 (1996).
5. Liu, P. et al. Age-related differences in memory-encoding fMRI responses after accounting for decline in vascular reactivity. Neuroimage 78, 415–25 (2013).
6. Leung, J., Kosinski, P. D., Croal, P. L. & Kassner, A. Developmental trajectories of cerebrovascular reactivity in healthy children and young adults assessed with magnetic resonance imaging. J. Physiol. 594, 2681–2689 (2016).
Figures