4626
Prediction of treatment response based on baseline volumetric functional MR imaging criteria in patients with unresectable HCC who are candidate for Trans-Arterial Chemoembolization.1John Hopkins Hospital, Baltimore, MD, United States, 2Johns Hopkins Hospital, Baltimore, MD, United States, 3Johns Hopkins Hospital, baltimore, MD, United States, 4johns Hopkins Hospital, Baltimore, MD, United States
Synopsis
Synopsis: Predicting overall survival in HCC patients before initiating treatment is essential to personalize a treatment plan for each patient. Barcelona clinic liver cancer (BCLC) is one of the current criteria for predicting pre-therapeutic overall survival (OS). Several studies suggested a valuable role of functional MRI biomarkers including diffusion-weighted imaging (DWI) with apparent diffusion coefficients (ADC), tumor venous enhancement (VE) and tumor volume (TV) in tumor response assessment. These metrics rely on changes post therapy; none of these parameters assessed baseline values of these variables in predicting OS before starting treatment.
Introduction
Introduction: Tumor response assessment after trans- arterial chemoembolization (TACE) plays an important role in determining an individualized treatment plan for each patient. Several standardized approaches have been proposed to assess tumor response which are mainly focused on change of tumor size after initial treatment 1,2. In this study we aim to stratify patients based on baseline imaging criteria to predict tumor response before trans- arterial chemoembolization (TACE) in patients with unresectable hepatocellular carcinoma (HCC).Methods
Methods: In this retrospective, single center, IRB-approved and HIPPA compliant study 150 patients with pathologically proven unresectable HCC were included. Treatment plan for all the cases was either conventional or DEB-TACE. All cases had baseline functional MRI. Average of 2 years follow up information was available for all cases. Imaging criteria including tumor volume, volumetric portal venous phase enhancement and volumetric diffusion-weighted imaging with apparent diffusion coefficients (ADC). Volumetric measurements were obtained by hand-tracing of the entire tumor using a post processing MRI software (Onco Treat, 3.1.1, Siemens). Overall survival (OS) of patients was evaluated in a cox proportional hazard model which includes all imaging variables (base ADC, Enhancement and tumor volume) and demographic information (age, gender).Results
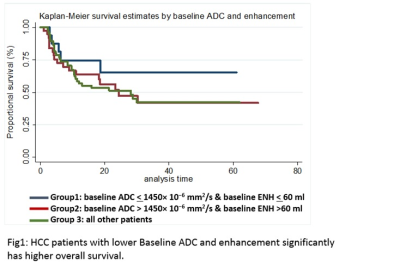
Results: Hazard ratio for baseline ADC and tumor volume showed significant association with patient OS. 1 ml increase in baseline tumor volume and 100 unit increase in ADC would result in 8% and 7% decrease OS, respectively. Hazard ration were not significant for baseline enhancement, age and gender. Patients were stratified into three groups based on significant baseline ADC and tumor volume for predicting OS ( group 1; patients with baseline ADC < 1450 × 10−6 mm2/s and baseline tumor volume < 60 ml, group 2; patients with baseline ADC > 1450 × 10−6 mm2/s and tumor volume > 60 ml, group 3; all other patients). Kaplan-Meier survival curve showed statistically significant improved OS in group 1(with lower baseline ADC and lower baseline tumor volume) compared to group 2 and 3 (Fig 1)Discussion
Discussion: HCC patients who undergo TACE therapy with baseline ADC < 1450× 10−6 mm2/s and baseline tumor volume < 60 ml (regardless of volume of enhanced tumor, age and gender) were more likely to survive in comparison to patients with baseline ADC > 1450× 10−6 mm2/s and tumor volume < 60 ml. A novel survival model that includes baseline volumetric ADC and tumor volume in addition to current BCLC criteria could potentially assist in patient selection prior to TACE/DEB TACE.Conclusion
Conclusion: Baseline volumetric ADC and tumor volume can assist in patient selection prior to TACE/DEB TACE and predicting OS. This could also help improve patient OS by avoiding invasive procedures and their side effects in patients who are unlikely to respond to them. Such novel clinical and functional imaging model could be applied in future clinical trials to better assess its efficacy.Acknowledgements
I do not have any acknowledgment.References
1. Kim MN, Kim BK, Han KH, et al. Evolution from WHO to EASL and mRECIST for hepatocellular carcinoma: considerations for tumor response assessment. Expert Rev Gastroenterol Hepatol. 2015 Mar;9(3):335-48
2. Edeline J, Boucher E, Rolland Y, et al. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012 Jan 1;118(1):147-56.